GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
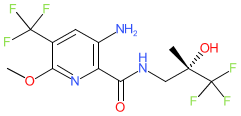
Synonyms: compound 33 [PMID: 34028270] | QBW-251 | QBW251
Compound class:
Synthetic organic
Comment: Icenticaftor (QBW251) is a compound that potentiates/improves transport of chloride ions via mutant cystic fibrosis transmembrane conductance regulator (CFTR) ion channels [1]. It is a clinical lead for cystic fibrosis and chronic obstructive pulmonary disease (COPD) [2], having demonstrated clinical proofs of concept in both conditions. In patients with CF it induced significant clinical benefit in those with Class III and IV CFTR mutations. Class III and IV mutations are those which impair gating or conductance of the channel respectively [3]. The potentiator ivacaftor is aleady approved to restore function in channels with class III mutations, but at the time of writing (May 2021) there was no approved potentiator for class IV mutant channels.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02190604 | Safety, Tolerability, Pharmacokinetics, and Preliminary Pharmacodynamics of QBW251 in Healthy Subjects and Cystic Fibrosis Patients | Phase 1/Phase 2 Interventional | Novartis | 2 | |
| NCT04268823 | Clinical Study to Assess the Mode of Action of QBW251 in Patients With Chronic Obstructive Pulmonary Disease (COPD) | Phase 2 Interventional | Novartis | ||
| NCT04072887 | Dose-range Finding Efficacy and Safety Study for QBW251 in COPD Patients | Phase 2 Interventional | Novartis | ||
| NCT02449018 | A Safety, Tolerability and Efficacy Study With QBW251 in COPD Patients With QBW251 | Phase 2 Interventional | Novartis | ||