|
Synonyms: AP-1189 | example 19 [WO2007141343A1]
Compound class:
Synthetic organic
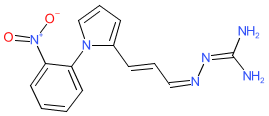
Comment: AP1189 is a biased agonist of melanocortin (MC) receptors. It was designed to elicit the proresolving properties of the MC1 and MC3 receptors in inflammation, whilst sparing their melanogenic effects [2]. This is achieved by bias towards ERK1/2 phosphorylation and and Ca2+ mobilisation rather than inducing canonical cAMP production. The chemical structure is claimed in Action Pharma's patent WO2007141343A1 (compound 2/example 19) [1].
SARS-CoV-2 and COVID-19: The proresolving activity of AP1189 is being investigated as a mechanism to combat the dysfunctional immune response in COVID-19 patients. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04456816 | A Study of the Safety, Tolerability, Pharmacokinetics and Efficacy of Treatment With AP1189 in Patients With iMN | Phase 2 Interventional | SynAct Pharma Aps | ||
| NCT04004429 | A Study of the Safety, Tolerability and Efficacy of Treatment With AP1189 in Early RA Patients With Active Joint Disease | Phase 2 Interventional | SynAct Pharma Aps | ||