|
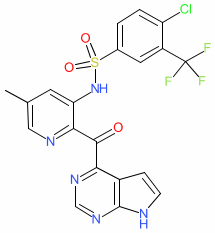
Synonyms: CCX-140B | CCX140 | CCX140-B | CCX140B | Example 5 [WO2009009740A1]
Compound class:
Synthetic organic
Comment: Ilacirnon (CCX140B) is a clinical stage, orally administered antagonist of the chemokine receptor CCR2 [3]. It was developed by Chemocentryx. The chemical structure of CCX140B matches that submitted to the WHO for the INN ilacirnon, and is one of those claimed in Chemocentryx's patent WO2009009740A1 [2]
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| CCX140 was advanced to clinical evaluation, reaching Phase 2 for diabetic nephropathy and the rare kidney disorder focal segmental slomerulosclerosis (FSGS). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01440257 | A Study to Evaluate the Effect of CCX140-B on Urinary Albumin Excretion in Subjects With Type 2 Diabetes and Albuminuria | Phase 2 Interventional | ChemoCentryx | ||
| NCT03703908 | A Study of CCX140-B in Subjects With Primary FSGS and Nephrotic Syndrome | Phase 2 Interventional | ChemoCentryx | ||
| NCT01447147 | A Study to Evaluate the Safety and Efficacy of CCX140-B in Subjects With Diabetic Nephropathy | Phase 2 Interventional | ChemoCentryx | 1 | |
| NCT03536754 | A Study of CCX140-B in Subjects With FSGS | Phase 2 Interventional | ChemoCentryx | This trial is known as the LUMINA-1 study. In mid-2020, Chemocentryx announced (on their company website) that this study had found no evidence of meaningful clinical efficacy in FSGS patients compared to placebo after 12 weeks of CCX140 treatment, and that the compound would not continue in FSGS. | |