|
Synonyms: AR-100 | AR100 | Mersarex® | RO-48-2622 | RO-482622
Compound class:
Synthetic organic
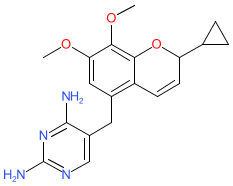
Comment: Iclaprim is a dihydrofolate reductase (DHFR) inhibitor that reduces folic acid synthesis. It is a diaminopyrimidine antibacterial that has broad-spectrum activity against Gram +ve pathogens [1]. Iclaprim is a racemic mixture of stereoisomers. We show the 'flat' structure to represent the mixture.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Iclaprim was developed to treat acute bacterial skin and skin structure infections (ABSSSI) and hospital-acquired bacterial pneumonia[4]. Phase 3 trials comparing iclaprim against both vancomycin and linezolid for ABSSSI have been completed. An application for marketing authorisation to the EMA for Mersarex, to be used to treat complicated skin and soft tissue infections, was withdrawn in 2009 [3]. A New Drug Application (from Motif Bio) for treatment of ABSSSI was accepted for review by the FDA in 2018. The FDA's response to this NDA stipulated that more data, and additional clinical study, addressing liver toxicity risk would be required before it could be approved [2]. |