|
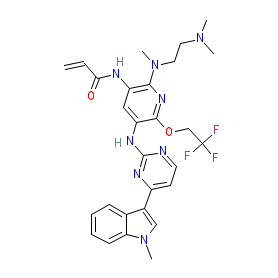
Synonyms: alflutinib (pseudo INN) | ASK120067 | AST-2818 | AST2818 | example 3 [US10072002B2] | furmonertinib | Ivesa®
firmonertinib is an approved drug (China (2021))
Compound class:
Synthetic organic
Comment: Firmonertinib (AST2818; formerly furmonertinib) is a receptor tyrosine kinase inhibitor that targets mutant forms of the EGFR more effectively than the wild type receptor kinase, with notable potency vs. the T790M resistance mutation. Firmonertinib is the formal WHO INN. It has an irreversible binding mode. AST2818's chemical structure is claimed as example 3 in Shanghai Allist Pharmaceuticals' patent US10072002B2 [2]. Note that AST2818 is administered clinically as the mesylate.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| This inhibitor was advanced to Phase 3 evaluation in patients with locally advanced/metastatic non-small cell lung cancer with EGFR sensitising mutations. Click here to link to ClinicalTrials.gov's full list of alflutinib studies. Furmonertinib was first approved for clinical use in China in 2021 [1]. It is indicated for the treatment of patients with locally advanced/metastatic NSCLC with confirmed EGFR T790M mutation, and whose disease has progressed during or after prior EGFR tyrosine kinase inhibitor (TKI) therapy. |