GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
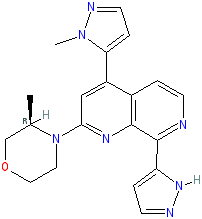
Synonyms: BAY-1895344 | BAY1895344
Compound class:
Synthetic organic
Comment: Elimusertib (BAY1895344) is a potent, highly selective and orally available ATR inhibitor. It was designed using a combination of medicinal chemistry, pharmacology, DMPK (drug metabolism and pharmacokinetics) and computational chemistry. ATR responds to a broad spectrum of DNA damage and activates the DNA damage response (DDR) pathway. By preventing DNA repair in cancer cells (which are under replication stress and suffer from increased DNA damage and defective DNA damage repair), inhibition of ATR is expected to drive the cancer cells towards cell cycle arrest and cell death [2-3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Click here to link to ClinicalTrials.gov's full list of BAY1895344 trials. Preliminary first-in-human data for BAY1895344 was presented at the 2019 ASCO Annual Meeting (see De Bono et al., Abstract No: 3007). These results show that BAY1895344 was tolerated at biologically active doses, and that it exhibited anti-tumour efficacy in solid tumours with DDR defects (clinical trial NCT03188965). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03188965 | First-in-human Study of ATR Inhibitor BAY1895344 in Patients With Advanced Solid Tumors and Lymphomas | Phase 1 Interventional | Bayer | 4 | |