GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: AC0010 | AC0010MA | avitinib (superceded INN) | FUJOVEE®
Compound class:
Synthetic organic
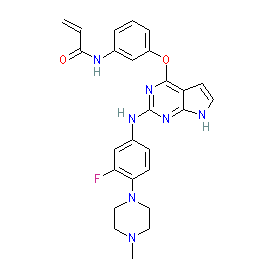
Comment: Abivertinib (AC0010) is an orally active, irreversible EGFR inhibitor (third generation) that selectively targets mutated EGFRs, and which was designed to overcome T790M-induced resistance in tumours [2,4]. It also inhibits BTK, thus offering extended clinical utility [3]. We show the structure for the free base form of the compound. The maleate salt formulation is known as STI-5656.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Clinical efficacy of AC0010 as an anti-cancer candidate is being evaluated in a number of clinical trials. Phase 1 results in NSCLC were published in mid 2018 [1]. The most advanced trial is a Phase 3 study that is designed to compare AC0010 and chemotherapy (pemetrexed/cisplatin) in patients with advance NSCLC whose disease has progressed following prior EGFR inhibitor therapy (NCT03058094). COVID-19: Abivertinib maleate (STI-5656) was evaluated for clinical efficacy in hospitalised COVID-19 patients. This trial was assessing the utility of exploiting the BTK-mediated immunomodulatory action of abivertinib, that inhibits production of key pro-inflammatory cytokine production, including IL-1β, IL-6 and TNFα, all of which are associated with cytokine release syndrome/cytokine storm, poor outcomes and disease progression in hospitalised COVID-19 patients. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03058094 | A Study Comparing AC0010 and Chemotherapy in Patients With Advanced NSCLC Who Have Progressed Following Prior EGFR TKI | Phase 3 Interventional | Hangzhou ACEA Pharmaceutical Research Co., Ltd. | ||
| NCT04440007 | Study of the Efficacy and Safety of STI-5656 (Abivertinib Maleate) in Subjects Hospitalized With COVID-19 | Phase 2 Interventional | Sorrento Therapeutics, Inc. | ||
| NCT04528667 | Study of the Safety and Efficacy of STI-5656 (Abivertinib Maleate) in Subjects Hospitalized Due to COVID-19 | Phase 2 Interventional | Sorrento Therapeutics, Inc. | ||
| NCT02330367 | Safety, Pharmacokinetic and Preliminary Efficacy Study of AC0010 in Patients With EGFR T790M Positive NSCLC | Phase 1/Phase 2 Interventional | Hangzhou ACEA Pharmaceutical Research Co., Ltd. | 4 | |