GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
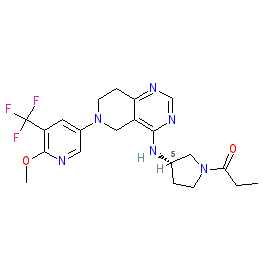
Synonyms: CDZ-173 | CDZ173 | Example 67 [WO2012004299] | Joenja®
leniolisib is an approved drug (FDA (2023))
Compound class:
Synthetic organic
Comment: Leniolisib (CDZ173) is an orally bioavailable, potent phosphatidylinositol 3-kinase inhibitor (PI3K) inhibitor, with selectivity for the PI3Kδ isoform [3]. Novartis originally developed CDZ173 as a treatment for autoimmune diseases, but has rapidly repurposed it for its potential in patients with Activated PI3K Delta Syndrome (APDS, a.k.a. p110 delta activating mutation causing senescent T cells, lymphadenopathy and immunodeficiency, or PASLI). APDS is an ultra-rare genetic immunodeficiency disease characterised by lymphoproliferation, recurrent infections from childhood, and an increased risk of EBV-associated lymphoma. APDS is known to be caused by autosomal dominant, gain-of-function mutations in the PIK3CD gene which encodes the PI3Kδ protein [4,7].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Data are presented for Example 67 in patent WO2012004299 [1]. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||