GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: (R)-crizotinib | PF 2341066 | PF-02341066 | PF-2341066 | PF2341066 | Xalkori®
crizotinib is an approved drug (FDA (2011), EMA (2012))
Compound class:
Synthetic organic
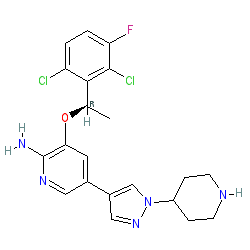
Comment: Critzotinib is a Type-1 kinase inhibitor and was first approved by the FDA in 2011. It inhibits ALK, cMET and ROS1 receptor tyrosine kinases. Critzotinib is a chiral molecule that exploits the three-dimensional space of the ATP pocket to achieve optimal potency and selectivity [6]. The R-enantiomer as shown here exhibits better potency than the either the racemate or S-isomer and the approved drug should contain only the R-enantiomer.
Pfizer developed the 3rd generation ALK inhibitor lorlatinib as a follow-up to critzotinib, and this new drug has been reported to outperfom its predecessor in lung cancer. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| The (S)-enantiomer ((S)-crizotinib) is considerably less potent than the (R)-enantiomer against its established protein kinase targets [4]. Interestingly, (S)-crizotinib has been reported to inhibit the activity of NUDT1 (P36639) with a Kd of 48nM [4]. NUDT1 is an antimutagenic enzyme involved in the effective elimination of oxidised nucleotides (induced by oxidative stress, for example), thus preventing their incorporation into DNA. At this target the (R)-enantiomer is the less potent enantiomer (Kd 781nM). This highlights the necessity that drug compounds be steroespecifically pure when there is a pharmacological difference in activity between stereoisomers (ie they exhibit distinct molecular mechanisms of action), as enantiomerically impure mixtures may result in off-target effects. A number of chemical suppliers and other submitters to online chemistry databases specify the non-stereoisomeric molecule depicted in PubChem CID 11597571. |
| Selectivity at catalytic receptors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Targets where the ligand is described in the comment field | |
| Target | Comment |
| ALK receptor tyrosine kinase | Crizotinib appears to be a selective ALK inhibitor acting on the tyrosine kinase activity [3] |
| Ligand mentioned in the following text fields |
| Type XVII RTKs: ROS receptors comments |