|
Synonyms: lasidonin | rubescensin A
Compound class:
Natural product or derivative
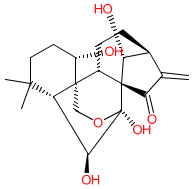
Comment: Oridonin is a plant-derived terpenoid. It has been identified as an active ingredient in species including Rabdosia rubescens (Chinese sage) [12], Isodon rugosus and Isodon ternifolius. Oridonin is proposed to exhibit a wide range of pharmacological properties, including antineoplastic, anti-angiogenic, pro-apoptotic, anti-asthmatic, anti-inflammatory and anti-bacterial actions [1]. Rabdosia rubescens root extract has been used in traditional Chinese medicine to treat inflammatory diseases and oral cancer.
The dissection of oridonin's specific mechanism of action and identification of its molecular target(s) is ongoing. In respect of inflammation, oridonin binds covalently to NLRP3 and blocks its interaction with NEK7, which ultimately prevents NLRP3 inflammasone assembly and activation [2]. It does not suppress activation of NLRC4 or AIM2 inflammasomes. Oridonin also inhibits AKT serine/threonine kinases in vitro, which may be associated with its anti-tumour activity [8-9]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Experimental evidence suggests that oridonin acts as a covalent NLRP3 inhibitor [2], with anti-inflammatory activity reported in a range of inflammation-driven human disease models [3-4,6,10-11], and has antiviral actions [5]. Anti-SARS-CoV-2 potential is proposed via oridonin binding to the coronavirus non-structural protein 9 (nsp9; Kd 7.2 μM) [7]. |
| Selectivity at enzymes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Selectivity at catalytic receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||