GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
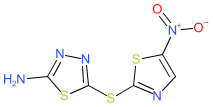
Synonyms: compound 9 [PMID: 19271755] | halicin | SU3327
Compound class:
Synthetic organic
Comment: SU3327 was originally identified as an inhibitor of the serine/threonine c-Jun N-terminal kinases (JNKs) [2]. It is substrate competitive (inhibiting the protein-protein interaction between JNK and the substrate JIP-1), and selective for JNK vs. p38 MAPK and Akt. SU3327 was shown to restore insulin sensitivity in mouse models of diabetes.
In the search for new antibacterials, artificial intelligence algorithms were used to identify novel chemotypes that were likely to have novel mechanisms of antibacterial activity. SU3327 (re-named as halicin) was identified by this process and in vitro and in vivo follow-up showed that it potently killed a wide range of difficult to treat, antibacterial-resistant bacterial strains [3]. The compound appears to disrupt the ability of bacteria to maintain an electrochemical gradient across their cell membranes which blocks their production of ATP for energy. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| SU-3327 demonstrates in vitro activity against both Gram-positive and Gram-negative bacteria. The MIC for antibacterial activity is 16 μg/ml for Staphylococcus aureus, 32 μg/ml for Escherichia coli, 128 μg/ml for Acinetobacter baumannii, and 256 μg/ml for multidrug-resistant (MDR) clinical isolates of Acinetobacter baumannii [1]. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||