GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
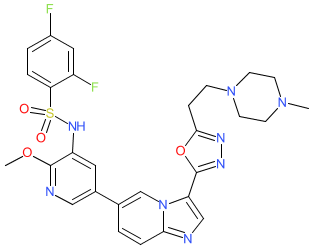
Comment: Compound 15a is a dual phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor that was designed for potential anti-tumour activity [1]. Dysregulation of the PI3K-Akt-mTOR signalling pathway is often observed in cancers, and simultaneous inhibition of PI3K and mTOR is predicted to offer improved antitumour activity compared to drugs that target single components of the pathway. A number of dual PI3K/mTOR inhibitors are in clinical development (for example gedatolisib, bimiralisib, apitolisib, samotolisib and omipalisib). All of these inhibitors are associated with adverse effects. Compound 15a was designed to address issues around selectivity and toxicity. Unfortunately 15a produced hepatic toxicity in vivo, so further optimisation would be essential to identify a more suitable derivative.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Compound 15a inhibits PI3Kα in HCT116 cells with an IC50 value of 10 nM [1]. In a DiscoveRX kinase screen against 97 kinases, only 3 hits for 15a were detected; PI3Kα, PI3Kγ (both class I PI3Ks) and the class II enzyme PIK3C2B. Oral administration reduced tumour proliferation in HCT116 and HT-29 xenografts, but its effects were no better than injections of copanlisib in these models. Liver toxicity was observed in the test animals. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||