GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
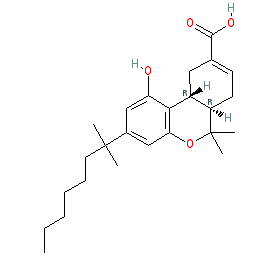
Synonyms: ajulemic acid | CPL-7075 | CPL7075 | CT-3 | IP-751 | IP751
Compound class:
Synthetic organic
Comment: Lenabasum (ajulemic acid) is a synthetic derivative of the non-psychoactive cannabinoid THC metabolite 11-nor-9-Carboxy-THC (PubChem CID 108207) [10]. It is a non-selective cannabinoid receptor agonist [5,7]. Lenabasum exhibits potentially useful analgesic and anti-inflammatory actions [3].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ajulemic acid (research code JBT-101) is being evaluated as a treatment for systemic lupus erythematosus (SLE) in Phase 2 clinical trial NCT03093402. It was also more effective in reducing chronic neuropathic pain than placebo in a Phase 2 clinical trial [6]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03093402 | JBT-101 in Systemic Lupus Erythematosus (SLE) | Phase 2 Interventional | National Institute of Allergy and Infectious Diseases (NIAID) | ||
| NCT02466243 | Safety, Tolerability, and Efficacy of JBT-101 in Subjects With Dermatomyositis | Phase 2 Interventional | Corbus Pharmaceuticals Inc. | Primary completion of this trial is expected in September 2021, with full completion a year later. Data is likely to be shared by the end of 2021. | |
| NCT03398837 | Trial to Evaluate Efficacy and Safety of Lenabasum in Diffuse Cutaneous Systemic Sclerosis | Phase 3 Interventional | Corbus Pharmaceuticals Inc. | Failure of lenabasum in this Phase 3 study in cutaneous systemic sclerosis patients was advised in September 2020. | |
| NCT02465437 | Safety, Tolerability, Efficacy, and Pharmacokinetics of JBT-101 in Systemic Sclerosis | Phase 2 Interventional | Corbus Pharmaceuticals Inc. | 8 | |
| NCT02465450 | Safety, Tolerability, Pharmacokinetics, and Efficacy of JBT-101 (Lenabasum) in Cystic Fibrosis | Phase 2 Interventional | Corbus Pharmaceuticals Inc. | Despite positive indications from this study lenabasum failed to do better than placebo at reducing pulmonary exacerbations in Phase 2b cystic fibrosis trial NCT03451045. Development in CF was subsequently discontinued. | 4 |