GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
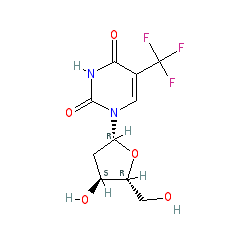
Synonyms: TFT | trifluorothymidine
trifluridine is an approved drug (Japan, FDA (2015), EMA (2016))
Compound class:
Synthetic organic
Comment: Trifluridine was originally approved by the US FDA in 1980, and was used for its antiviral effects (marketed as Vitroptic®). The drug is now being investigated for its antineoplastic (cytotoxic) effect.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Trifluridine is a component of the anticancer therapeutic Lonsurf® (code-named TAS-102), that was first approved in Japan for the treatment of metastatic/unresectable colorectal cancer. The other component of TAS-102 is tipiracil [1]. In September 2015, the US FDA approved Lonsurf® for the treatment of patients with RAS wild type, metastatic colorectal cancer who have already received chemotherapy, anti-VEGF, and anti-EGFR therapies. Lonsurf® was EMA approved in 2016. TAS-102 is being assessed in Phase 3 clinical trial for metastatic gastric cancer (NCT02500043). |
Mechanism Of Action and Pharmacodynamic Effects  |
| Trifluridine is a modified form of deoxyuridine (nucleoside analogue). Trifluridine is metabolised to its monophosphate-derivative and this inhibits thymidylate synthase, one of the rate-limiting enzymes in pyrimidine de novo deoxynucleotide synthesis. Thymidylate synthase is therefore crucial for DNA synthesis. In addition, if trifluridine is incorporated directly in to DNA during replication, the -CF3 group of trifluridine blocks base pairing. These actions underly the compound's cytotoxic effect [2]. |
External links  |
|
For extended ADME data see the following: Drugs.com |