GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
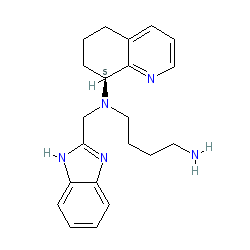
Synonyms: AMD 070 | AMD-070 | AMD-11070 | AMD11070 | compound 2 [PMID: 20297846] | X4P-001 | X4P-001-IO | X4P-001-LD | Xolremdi®
mavorixafor is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: Mavorixafor (AMD070/AMD11070) is a potent, selective and bioavailable CXCR4 chemokine receptor allosteric antagonist [5]. Originally developed for HIV treatment [4], it was repurposed by X4 Pharmaceuticals as X4P-001 for the treatment of WHIM syndrome, a sub-type of a primary immunodeficiency disease caused by CXCR4 mutations. The compound is exemplified in a process patent US7332605 and as compound 89 from a series of 169 analogues in WO2003055876 but neither filing includes activity data.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Badolato R, Alsina L, Azar A, Bertrand Y, Bolyard AA, Dale D, Deyà-Martínez À, Dickerson KE, Ezra N, Hasle H et al.. (2024)
A phase 3 randomized trial of mavorixafor, a CXCR4 antagonist, for WHIM syndrome. Blood, 144 (1): 35-45. [PMID:38643510] |
|
2. Dale DC, Bolyard AA, Makaryan V. (2023)
The promise of novel treatments for severe chronic neutropenia. Expert Rev Hematol, 16 (12): 1025-1033. [PMID:37978893] |
|
3. Hoy SM. (2024)
Mavorixafor: First Approval. Drugs, 84 (8): 969-975. [PMID:39004659] |
|
4. Moyle G, DeJesus E, Boffito M, Wong RS, Gibney C, Badel K, MacFarland R, Calandra G, Bridger G, Becker S et al.. (2009)
Proof of activity with AMD11070, an orally bioavailable inhibitor of CXCR4-tropic HIV type 1. Clin Infect Dis, 48 (6): 798-805. [PMID:19193109] |
|
5. Skerlj RT, Bridger GJ, Kaller A, McEachern EJ, Crawford JB, Zhou Y, Atsma B, Langille J, Nan S, Veale D et al.. (2010)
Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem, 53 (8): 3376-88. [PMID:20297846] |
|
6. Wong RS, Bodart V, Metz M, Labrecque J, Bridger G, Fricker SP. (2008)
Comparison of the potential multiple binding modes of bicyclam, monocylam, and noncyclam small-molecule CXC chemokine receptor 4 inhibitors. Mol Pharmacol, 74 (6): 1485-95. [PMID:18768385] |