GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
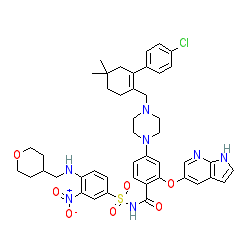
Synonyms: ABT-199 | GDC-0199 | Venclexta® | Venclyxto®
venetoclax is an approved drug (FDA and EMA (2016))
Compound class:
Synthetic organic
Comment: Venetoclax is a BH3 mimetic that selectively targets Bcl-2 [4], utilised for its pro-apoptotic activity. It is noteworthy that venetoclax is the first approved medicine designed to induce a natural process that helps cells self-destruct.
The IUPAC name provided by PubChem matches 'Example 5' claimed in patent US8580794 [1]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Venetoclax (in combination with and in comparison with currently approved anti-neoplastics) was assessed in Phase 3 clinical trials as a potential treatment for chronic lymphocytic leukemia (CLL). See NCT02005471 and NCT02242942 for full details. In January 2016, the US FDA granted venetoclax (in combination with hypomethylating agents) breakthrough designation as a first-line treatment for patients with acute myeloid leukemia (AML) who are ineligible to receive high-dose chemotherapy. Full marketing authorisation: In April 2016, the US FDA granted full approval for venetoclax as an oral treatment regimen for patients with chronic lymphocytic leukemia (CLL) with 17p deletion (or TP53 mutation) (detected by an FDA-approved test), who have received at least one prior therapy. EMA approval followed in December 2016. In June 2018, the FDA expanded approval to include treatment of both CLL and small lymphocytic lymphoma (SLL), with or without 17p deletion in patients who have received at least one prior therapy. This approval is for a combined regimen using venetoclax plus the anti-CD20 monoclonal rituximab [3], and offers a chemotherapy-free option for CLL patients. Venetoclax plus anti-CD20 obinutuzumab gained FDA approval for use in adults with CLL or SLL in May 2019, based on results from the CLL14 trial (NCT02242942). |
Mechanism Of Action and Pharmacodynamic Effects  |
| Venetoclax selectively inhibits the ability of the Bcl-2 protein to sequester its pro-apoptotic family members, thereby restoring the apoptotic balance in cells with aberrant Bcl-2 activity. Venetoclax does not inhibit Bcl-xL, so does not exhibit the anti-platelet side-effect of previously investigated non-selective Bcl-2 inhibitors (eg navitoclax; research code ABT-263). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02005471 | A Study to Evaluate the Benefit of Venetoclax Plus Rituximab Compared With Bendamustine Plus Rituximab in Participants With Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) | Phase 3 Interventional | Hoffmann-La Roche | ||
| NCT02242942 | A Study to Compare the Efficacy and Safety of Obinutuzumab + Venetoclax (GDC-0199) Versus Obinutuzumab + Chlorambucil in Participants With Chronic Lymphocytic Leukemia | Phase 3 Interventional | Hoffmann-La Roche | ||
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |