GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
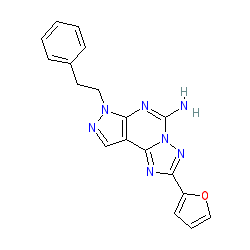
Synonyms: SCH-58261 | SCH58261
Compound class:
Synthetic organic
Comment: SCH 58261 is a selective adenosine A2A receptor antagonist used as a research tool compound [9]. Reports of its effect in models of Parkinson's disease have been published [2,7].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Beukers MW, den Dulk H, van Tilburg EW, Brouwer J, Ijzerman AP. (2000)
Why are A(2B) receptors low-affinity adenosine receptors? Mutation of Asn273 to Tyr increases affinity of human A(2B) receptor for 2-(1-Hexynyl)adenosine. Mol Pharmacol, 58 (6): 1349-56. [PMID:11093773] |
|
2. Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli Jr N, Schwarzschild MA. (2001)
Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci, 21 (10): RC143. [PMID:11319241] |
|
3. Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, Fredholm BB. (1997)
Characterization of human A2A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. Br J Pharmacol, 121 (3): 353-60. [PMID:9179373] |
|
4. Jacobson KA, Gao ZG. (2006)
Adenosine receptors as therapeutic targets. Nat Rev Drug Discov, 5 (3): 247-64. [PMID:16518376] |
|
5. Kull B, Arslan G, Nilsson C, Owman C, Lorenzen A, Schwabe U, Fredholm BB. (1999)
Differences in the order of potency for agonists but not antagonists at human and rat adenosine A2A receptors. Biochem Pharmacol, 57 (1): 65-75. [PMID:9920286] |
|
6. Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. (1999)
Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol, 359 (1): 7-10. [PMID:9933143] |
|
7. Simola N, Fenu S, Baraldi PG, Tabrizi MA, Morelli M. (2006)
Dopamine and adenosine receptor interaction as basis for the treatment of Parkinson's disease. J Neurol Sci, 248 (1-2): 48-52. [PMID:16780890] |
|
8. Townsend-Nicholson A, Schofield PR. (1994)
A threonine residue in the seventh transmembrane domain of the human A1 adenosine receptor mediates specific agonist binding. J Biol Chem, 269 (4): 2373-6. [PMID:8300561] |
|
9. Zocchi C, Ongini E, Conti A, Monopoli A, Negretti A, Baraldi PG, Dionisotti S. (1996)
The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist. J Pharmacol Exp Ther, 276 (2): 398-404. [PMID:8632302] |