GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
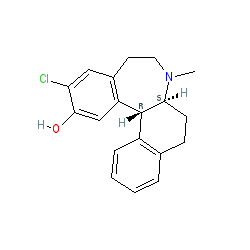
Synonyms: EBS-101 | PSYRX-101 | SCH 39166 | SCH-39166 | SCH39166
Compound class:
Synthetic organic
Comment: Ecopipam is an orally bioactive, brain penetrant dopamine receptor antagonist [4], with selectivity for the D1/5 subtypes [1]. It is structurally related to benzazepine. Ecopipam has been utilised to demonstrate (in animal models) the potential of D1 receptor antagonism to rescue autistic-like behaviours that are associated with the lysosomal storage disorder mucopolysaccharidosis type IIIA-D (MPS-IIIA-D) [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chipkin RE, Iorio LC, Coffin VL, McQuade RD, Berger JG, Barnett A. (1988)
Pharmacological profile of SCH39166: a dopamine D1 selective benzonaphthazepine with potential antipsychotic activity. J Pharmacol Exp Ther, 247 (3): 1093-102. [PMID:2905002] |
|
2. De Risi M, Cusimano L, Bujanda Cundin X, Pizzo M, Gigante Y, Monaco M, Di Eugenio C, De Leonibus E. (2025)
D1 dopamine receptor antagonists as a new therapeutic strategy to treat autistic-like behaviours in lysosomal storage disorders. Mol Psychiatry, 30 (7): 2980-2993. [PMID:39865184] |
|
3. Gilbert DL, Dubow JS, Cunniff TM, Wanaski SP, Atkinson SD, Mahableshwarkar AR. (2023)
Ecopipam for Tourette Syndrome: A Randomized Trial. Pediatrics, 151 (2). [PMID:36628546] |
|
4. Karlsson P, Sedvall G, Halldin C, Swahn CG, Farde L. (1995)
Evaluation of SCH 39166 as PET ligand for central D1 dopamine receptor binding and occupancy in man. Psychopharmacology (Berl), 121 (3): 300-8. [PMID:8584610] |
|
5. Panda PK, Panda P, Dawman L, Mishra AS, Kumar V, Sharawat IK. (2025)
Safety and Efficacy of Ecopipam in Patients with Tourette Syndrome: A Systematic Review and Meta-analysis. CNS Drugs, 39 (2): 127-142. [PMID:39730854] |
|
6. Sunahara RK, Guan HC, O'Dowd BF, Seeman P, Laurier LG, Ng G, George SR, Torchia J, Van Tol HH, Niznik HB. (1991)
Cloning of the gene for a human dopamine D5 receptor with higher affinity for dopamine than D1. Nature, 350 (6319): 614-9. [PMID:1826762] |
|
7. Tice MA, Hashemi T, Taylor LA, Duffy RA, McQuade RD. (1994)
Characterization of the binding of SCH 39166 to the five cloned dopamine receptor subtypes. Pharmacol Biochem Behav, 49 (3): 567-71. [PMID:7862709] |