GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
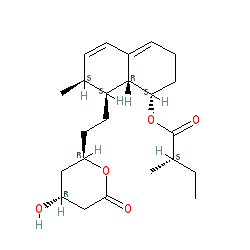
Comment: Mevastatin, originally isolated from Penicillium citrinum, was the first HMG-CoA reductase inhibitor (statin) to be identified [6].
Note, the structure presented here is a representative one, as there is some ambiguity in the stereoisomeric specification, see [2] and [5] for the primary references. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Baran JS, Laos I, Langford DD, Miller JE, Jett C, Taite B, Rohrbacher E. (1985)
3-Alkyl-3-hydroxyglutaric acids: a new class of hypocholesterolemic HMG CoA reductase inhibitors. 1. J Med Chem, 28 (5): 597-601. [PMID:3989819] |
|
2. Brown AG, Smale TC, King TJ, Hasenkamp R, Thompson RH. (1976)
Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J Chem Soc Perkin Trans I, (11): 1165-70. [PMID:945291] |
|
3. Coppola GM, Damon RE, Yu H, Engstrom RG, Scallen TJ. (1997)
Design and biological evaluation of a series of thiophene-based 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Bioorg Med Chem Lett, 7 (4): 549-554. |
|
4. Endo A. (1985)
Compactin (ML-236B) and related compounds as potential cholesterol-lowering agents that inhibit HMG-CoA reductase. J Med Chem, 28 (4): 401-5. [PMID:3981532] |
|
5. Endo A, Kuroda M, Tanzawa K. (1976)
Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett, 72 (2): 323-6. [PMID:16386050] |
|
6. Endo A, Kuroda M, Tsujita Y. (1976)
ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot, 29 (12): 1346-8. [PMID:1010803] |
|
7. Heathcock CH, Hadley CR, Rosen T, Theisen PD, Hecker SJ. (1987)
Total synthesis and biological evaluation of structural analogues of compactin and dihydromevinolin. J Med Chem, 30 (10): 1858-73. [PMID:3656359] |
|
8. Hosoda S, Matsuda D, Tomoda H, Hashimoto M, Aoyama H, Hashimoto Y. (2009)
Application of a 3,3-diphenylpentane skeleton as a multi-template for creation of HMG-CoA reductase inhibitors. Bioorg Med Chem Lett, 19 (15): 4228-31. [PMID:19502059] |
|
9. Roth BD, Blankley CJ, Chucholowski AW, Ferguson E, Hoefle ML, Ortwine DF, Newton RS, Sekerke CS, Sliskovic DR, Stratton CD. (1991)
Inhibitors of cholesterol biosynthesis. 3. Tetrahydro-4-hydroxy-6-[2-(1H-pyrrol-1-yl)ethyl]-2H-pyran-2-one inhibitors of HMG-CoA reductase. 2. Effects of introducing substituents at positions three and four of the pyrrole nucleus. J Med Chem, 34 (1): 357-66. [PMID:1992137] |
|
10. Sliskovic DR, Picard JA, Roark WH, Roth BD, Ferguson E, Krause BR, Newton RS, Sekerke C, Shaw MK. (1991)
Inhibitors of cholesterol biosynthesis. 4. trans-6-[2-(substituted-quinolinyl)ethenyl/ethyl]tetrahydro-4-hydroxy-2 H-pyran-2-ones, a novel series of HMG-CoA reductase inhibitors. J Med Chem, 34 (1): 367-73. [PMID:1992138] |
|
11. Stokker GE, Hoffman WF, Alberts AW, Cragoe EJ, Deana AA, Gilfillan JL, Huff JW, Novello FC, Prugh JD, Smith RL. (1985)
3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. 1. Structural modification of 5-substituted 3,5-dihydroxypentanoic acids and their lactone derivatives. J Med Chem, 28 (3): 347-58. [PMID:3973903] |
|
12. Thilagavathi R, Kumar R, Aparna V, Sobhia ME, Gopalakrishnan B, Chakraborti AK. (2005)
Three-dimensional quantitative structure (3-D QSAR) activity relationship studies on imidazolyl and N-pyrrolyl heptenoates as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) inhibitors by comparative molecular similarity indices analysis (CoMSIA). Bioorg Med Chem Lett, 15 (4): 1027-32. [PMID:15686906] |