GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
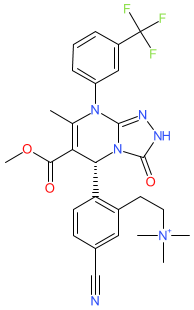
Synonyms: CHF 6333 | CHF-6333 | Example 47 (cation) [US9828382] [1]
Compound class:
Synthetic organic
Comment: CHF6333 is a lung-restricted (by inhalation) neutrophil elastase (NE) inhibitor [2]. It is formulated as a salt (e.g., as the methanesulfonate), and strictly this entry represents the CHF6333 cation. Local delivery to the lung is intended to minimise systemic exposure and off-target toxicity. NE inhibition is proposed to rebalance elevated NE activity that underlies disruption of vascular endothelium, vascular permeability and tissue damage in chronic inflammatory lung disease.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| CHF6333 is a clinical candidate that is proposed for the treatment of bronchiectasis. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03056326 | A Study to Investigate Safety, Tolerability and Pharmacokinetics of Single and Repeat Doses of CHF6333 in Healthy Subjects | Phase 1 Interventional | Chiesi Farmaceutici S.p.A. | ||
| NCT04010799 | A Clinical Study to Investigate Safety, Tolerability and Distribution of CHF 6333 After One or After Repeated Inhalation in Patients With Cystic Fibrosis (CF) and in Patients With Non Cystic Fibrosis (NCFB) Bronchiectasis | Phase 1 Interventional | Chiesi Farmaceutici S.p.A. | ||
| NCT06166056 | A Study to Investigate the Safety, Tolerability, and Pharmacokinetics of Inhaled CHF6333 After Single Doses in Healthy Volunteers and After Single and Repeated Doses in Subjects With Bronchiectasis | Phase 1/Phase 2 Interventional | Chiesi Farmaceutici S.p.A. | ||