GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
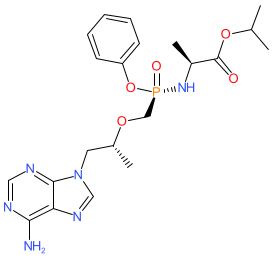
Synonyms: GS-7340 | GS7340 | Vemlidy®
tenofovir alafenamide is an approved drug
Compound class:
Synthetic organic
Comment: Tenofovir alafenamide is a tenofovir prodrug.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Schwiebert E, Wang Y, Xi R, Choma K, Streiff J, Flammer LJ, Rivers N, Ozdener MH, Margolskee RF, Christensen CM et al.. (2021)
Inhibition of Bitter Taste from Oral Tenofovir Alafenamide. Mol Pharmacol, 99 (5): 319-327. [PMID:33824185] |