GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
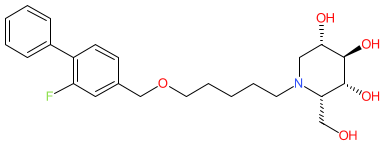
Synonyms: AZ-3102 | AZ3102 | compound 76 [PMID: 25250725]
Compound class:
Synthetic organic
Comment: Nizubaglustat (AZ-3102) is an azosugar (formerly called iminosugars). It is orally bioavailable and can cross the blood-brain barrier. Mechanistically it acts as an inhibitor of lysosomal and non-lysosomal enzymes that regulate ceramide turnover [1]. This approach is proposed for disease-modifying potential in the treatment of GM1 or GM2 gangliosidoses (neurodegenerative lysosomal storage diseases), and is exemplified by the approval of miglustat to treat Gaucher disease.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Nizubaglustat is a clinical lead for the treatment of GM2 gangliosidoses (Tay-Sachs or Sandhoff diseases) and Niemann-Pick disease type C (NP-C). The agent has orphan drug designations dating from 2022/3, from the EU EMA and US FDA for the treatment of GM2 gangliosidoses and NP-C. Around the same time, the UK HMRA issued an "Innovation Passport" for the treatment of GM1 and GM2 gangliosidoses. Tolerability, PK and PD results from a first-in-(healthy)human study completed in Australia (registry number ACTRN12621000576820) were published in 2024 [2]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05758922 | Phase 2 Study Evaluating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Oral AZ-3102 in Patients with GM2 Gangliosidosis or Niemann-Pick Type C Disease | Phase 2 Interventional | Azafaros A.G. | ||