GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
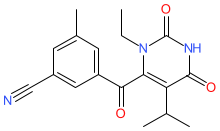
Synonyms: compound 10b [PMID: 20138513] | GS-9441 [3] | KM-023 | KM023
ainuovirine is an approved drug
Compound class:
Synthetic organic
Comment: Ainuovirine (KM-023) is a non-nucleoside reverse-transcriptase inhibitor (NNRTI) class antiretroviral that is intended to treat human immunodeficiency virus (HIV) type 1 infection [1-2].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The Chinese drug regulator (NMPA) approved the triple combination of ainuovirine, lamivudine and tenofovir (ANV) as an HIV anti-retroviral therapy in September 2024. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05420246 | Efficacy and Safety of Ainuovirine Treating With AIDS Patients | Observational | Guangzhou 8th People's Hospital | ||
| NCT01348516 | Single Ascending Dose (SAD)/Multiple Ascending Dose(MAD) Safety/Pharmacokinetic (PK) Study of KM-023 | Phase 1 Interventional | Kainos Medicine Inc. | 1 | |