GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
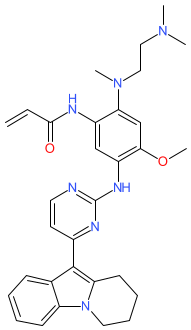
Synonyms: oritinib | rilertinib | SH1028
SH-1028 is an approved drug
Compound class:
Synthetic organic
Comment: SH-1028 (oritinib/rilertinib) is an irreversible third-generation EGFR tyrosine kinase inhibitor that retains efficacy against EGFRT790M-mediated resistance [1]. Neither oritinib or rilertinib are registered with the WHO as INNs, so we have not used these as primary name for the compound. We will review this as/when the structure can be matched to an INN.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The Chinese drug regulator (NMPA) approved oritinib/rilertinib (Saint Rasa®) in Jun 2024, to treat EGFRT790M mutated non-small cell lung cancer [4]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04810533 | The Absorption, Metabolism and Excretion of [14C]SH-1028 in Chinese Healthy Male Volunteers | Phase 1 Interventional | Nanjing Sanhome Pharmaceutical, Co., Ltd. | ||
| NCT06080776 | SH-1028 Tablets Versus Placebo as Adjuvant Therapy in Resected Stage II-IIIB NSCLC With Sensitizing EGFR Mutations | Phase 3 Interventional | Nanjing Sanhome Pharmaceutical, Co., Ltd. | ||
| NCT03823807 | A Study to Evaluate the Safety and Efficacy of SH-1028 in Locally Advanced or Metastatic NSCLC | Phase 2 Interventional | Nanjing Sanhome Pharmaceutical, Co., Ltd. | 3 | |
| NCT03603262 | Safety, Tolerability and Pharmacokinetics of SH-1028 in Patients With Advanced NSCLC | Phase 1 Interventional | Nanjing Sanhome Pharmaceutical, Co., Ltd. | 2 | |