GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
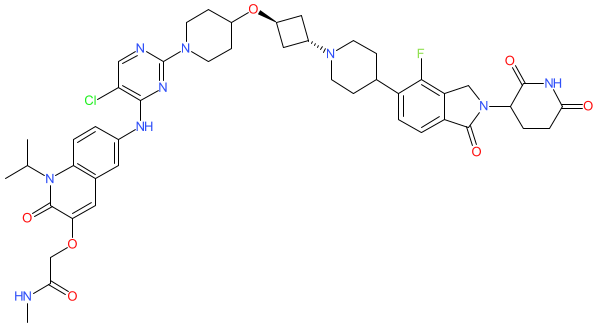
Synonyms: ARV-393 | ARV393 | compound 81 [WO2022221673A1]
Compound class:
Synthetic organic
Comment: ARV-393 is an orally active PROTAC that promotes degradation of the BCL6 transcription repressor protein. A lenalidomide derived moiety promotes formation of a ternary complex of the PROTAC, target protein and the E3 ligase cereblon to induce ubiquitination and degradation. The structure shown here has (1r,3r) configuration around the cyclobutoxy group, as indicated for compound 81 in Arvinas' patent WO2022221673A [1]. We will update this entry whenever ARV-393's chemical structure is formally disclosed.
BCL6 is a transcriptional repressor and major driver of B-cell lymphomas. Reducing BCL6 activity or abundance are proposed as mechanisms to treat advanced B-cell malignancies such as non-Hodgkin's lymphoma. In July 2025 we were able to match the structure of ARV-393 to the INN zaloblideg that was included in WHO INN proposed list 133. |
|
|||||||||||||||||||||||||||||||||||
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
| InChI standard identifier | Download |
| InChI standard key | Download |
Molecular structure representations generated using Open Babel