GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 1 [PMID: 32053979] | compound 5 [PMID: 22625994]
Compound class:
Synthetic organic
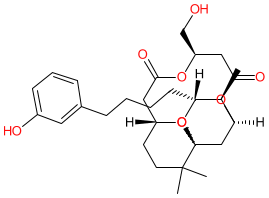
Comment: 10-Me-Aplog-1 is a PKC serine/threonine kinases activator [2-3]. Structurally it is a derivative of the marine mollusc toxin and tumour-promoting substance debromoaplysiatoxin [1]. In contrast to the parental toxin, 10-Me-Aplog-1 exhibits anti-proliferative activity in vitro, with no significant tumour-promoting or pro-inflammatory activities [2,4].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Hanaki Y, Shikata Y, Kikumori M, Hotta N, Imoto M, Irie K. (2018)
Identification of protein kinase C isozymes involved in the anti-proliferative and pro-apoptotic activities of 10-Methyl-aplog-1, a simplified analog of debromoaplysiatoxin, in several cancer cell lines. Biochem Biophys Res Commun, 495 (1): 438-445. [PMID:29129688] |
|
2. Kikumori M, Yanagita RC, Tokuda H, Suzuki N, Nagai H, Suenaga K, Irie K. (2012)
Structure-activity studies on the spiroketal moiety of a simplified analogue of debromoaplysiatoxin with antiproliferative activity. J Med Chem, 55 (11): 5614-26. [PMID:22625994] |
|
3. Murakami K, Yoshimura M, Nakagawa S, Kume T, Kondo T, Inoue H, Irie K. (2020)
Evaluation of Toxic Amyloid 42 Oligomers in Rat Primary Cerebral Cortex Cells and Human iPS-derived Neurons Treated with 10-Me-Aplog-1, a New PKC Activator. Int J Mol Sci, 21 (4). [PMID:32053979] |
|
4. Nakagawa Y, Yanagita RC, Hamada N, Murakami A, Takahashi H, Saito N, Nagai H, Irie K. (2009)
A simple analogue of tumor-promoting aplysiatoxin is an antineoplastic agent rather than a tumor promoter: development of a synthetically accessible protein kinase C activator with bryostatin-like activity. J Am Chem Soc, 131 (22): 7573-9. [PMID:19449873] |