GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
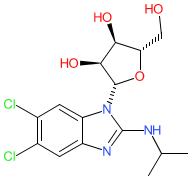
Synonyms: 1263W94 | benzimidavir | Camvia® | G1263 | GW-1263 | Livtencity®
maribavir is an approved drug (FDA (2021), EMA (2022))
Compound class:
Synthetic organic
Comment: Maribavir is an orally bioavailable benzimidazole L-riboside that inhibits cytomegalovirus (CMV) pUL97 kinase with nM potency in vitro [1]. It inhibits viral replication.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| In the US, maribavir is approved to treat refractory post-transplant CMV infection [2]. It is indicated for CMV infections that are resistant to ganciclovir, valganciclovir, cidofovir or foscarnet. In the EU maribavir can be used under orphan designation to treat drug-resistant CMV infections in patients who have impaired cell-mediated immunity. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02931539 | Efficacy and Safety Study of Maribavir Treatment Compared to Investigator-assigned Treatment in Transplant Recipients With Cytomegalovirus (CMV) Infections That Are Refractory or Resistant to Treatment With Ganciclovir, Valganciclovir, Foscarnet, or Cidofovir | Phase 3 Interventional | Takeda | ||
| NCT02927067 | A Study of Maribavir Compared to Valganciclovir to Treat Cytomegalovirus Infections in People Who Have Received Stem Cell Transplants | Phase 3 Interventional | Takeda | ||
| NCT01611974 | Maribavir for Treatment of Resistant or Refractory CMV Infections in Transplant Recipients | Phase 2 Interventional | Takeda | 4 | |
| NCT00411645 | Prophylactic Use of Maribavir for the Prevention of Cytomegalovirus (CMV) Disease in Stem Cell Transplant Recipients | Phase 3 Interventional | Takeda | 3 | |