GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: deuremidevir | JT001 | Mindavi® | VV-116 | VV116 | X6 hydrobromide [PMID: 34584244]
mindeudesivir is an approved drug (China NMPA (2023))
Compound class:
Synthetic organic
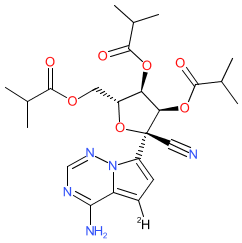
Comment: VV116 is an orally available, deuterated remdesivir analogue [6-7]. It is a tri-isobutyrate ester prodrug (formulated as the hydrobromide salt) that is converted to an active nucleoside triphosphate (116-NTP) that acts as a viral RNA-dependent RNA polymerase (RdRP) inhibitor. VV116 was developed for antiviral activity against SARS-CoV-2. We drew the chemical structure as represented in Xie et al. (2021) [6]. The chemical structure for VV116 was later matched to that for the INN mindeudesivir.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| VV116/JT001 was progressed to clinical evaluation as an early treatment for COVID-19. A phase I clinical trial of VV116 in China was completed [5], and more advanced trials are evaluating its efficacy and safety compared to the RdRp inhibitor favipiravir and the 3CLpro inhibitor nirmatrelvir (contained in Paxlovid®). The Chinese NMPA approved VV116 (deuremidevir hydrobromide; Mindavi®) in January 2023. It is also approved for use in Uzbekistan [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT05279235 | Efficacy and Safety of JT001 (VV116) Compared With Favipiravir | Phase 3 Interventional | Shanghai Vinnerna Biosciences Co., Ltd. | ||
| NCT05242042 | JT001 (VV116) for the Early Treatment of COVID-19 | Phase 2/Phase 3 Interventional | Shanghai JunTop Biosciences Co., LTD | ||
| NCT05341609 | Efficacy and Safety of JT001 (VV116) Compared With Paxlovid | Phase 3 Interventional | Vigonvita Life Sciences | 2 | |
| NCT05582629 | JT001 (VV116) for the Treatment of COVID-19 | Phase 3 Interventional | Shanghai Vinnerna Biosciences Co., Ltd. | 3 | |