GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
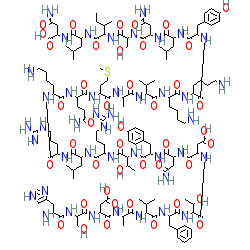
Endogenous peptide in human, mouse or rat
Comment: Sequences of human, mouse and rat VIP are identical
VIP was first isolated and sequenced from pig small intestine [12,15]. As with many peptide molecules, there is ambiguity surrounding representations of the exact stereochemistry of VIP. The structure shown here does not specify stereochemistry.
Species: Human, Mouse, Rat
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: vasoactive intestinal peptide |
|
|||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Clinical evaluation of VIP as an inhalation therapeutic for the treatment of pulmonary hypertension in COPD patients (NCT00464932) has been completed, but there is no indication of further development. Clinical use of native VIP is limited by its short circulating half-life and its gastrointestinal side-effects caused by activation of VPAC1 receptors which are found predominantly in the gastrointestinal tract. To overcome these limitations PHASEBio have developed a congugated VIP analogue that has an extended half-life (~60 hours) and selectivity for myocardial VPAC2 receptors. Coded as PB1046 (pemziviptadil INN; VasomeraTM), this synthetic VIP has advanced to Phase 2 clinical trial for evaluation as a once-weekly injection for patients with pulmonary arterial hypertension (see NCT03556020). In response to the SARS-CoV-2 pandemic and severe COVID-19 ARDS, PB1046 was entered into a clinical trial to evaluate its efficacy in COVID-19 patients at high risk of deterioration. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00464932 | Vasoactive Intestinal Peptide in COPD | Observational | Medical University of Vienna | ||
| NCT03556020 | Phase 2 Study to Assess Safety, Tolerability and Efficacy of Once Weekly SC PB1046 in Subjects With Symptomatic PAH | Phase 2 Interventional | PhaseBio Pharmaceuticals Inc. | ||
| NCT04433546 | Pemziviptadil (PB1046), a Long-acting, Sustained Release Human VIP Analogue, Intended to Provide Clinical Improvement to Hospitalized COVID-19 Patients at High Risk for Rapid Clinical Deterioration and Acute Respiratory Distress Syndrome (ARDS). | Phase 2 Interventional | PhaseBio Pharmaceuticals Inc. | ||