GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
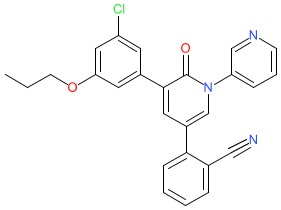
Comment: Compound 5 is a non-covalent SARS-CoV-2 Mpro (3CL-pro) inhibitor with in vitro antiviral activity [1]. It appears to provide synergistic antiviral activity with the viral polymerase inhibitor remdesivir in vitro, in a replicon assay that utilised a non-infectious SARS-CoV-2 clone. Structurally, 5 is a derivative of the anticonvulsant drug perampanel, and was designed to optimise perampanel's weak (IC50100-250 μM) Mpro inhibitory activity. Several nM potency inhibitors were identified in this study (e.g. 21, IC50 18 nM in an enzyme kinetic assay), but they did not deliver antiviral activity in the in vitro assays and/or were cytotoxic. Compound 26 with 1 μM antiviral activity and no cytotoxicity was highlighted as a significant lead structure. The crystal structure of 5 bound to SARS-CoV-2 Mpro has been resolved to 1.8 Å and was submitted to the Protein Data Bank with accession ID 7L11.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Zhang C-H, Stone EA, Deshmukh M, Ippolito JA, Ghahremanpour MM, Tirado-Rives J, Spasov KA, Zhang S, Takeo Y, Kudalkar SN et al.. (2021)
Potent Noncovalent Inhibitors of the Main Protease of SARS-CoV-2 from Molecular Sculpting of the Drug Perampanel Guided by Free Energy Perturbation Calculations. ACS Central Science,. DOI: 10.1021/acscentsci.1c00039 |