GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
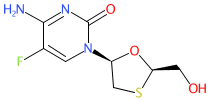
Abbreviated name: FTC
Synonyms: 524W91 | BW-524W91 | Coviracil® | Emtriva®
emtricitabine is an approved drug (EMA & FDA (2003))
Compound class:
Synthetic organic
Comment: Emtricitabine is a nucleoside reverse transcriptase inhibitor (NRTI) antiretroviral compound.

View more information in the IUPHAR Pharmacology Education Project: emtricitabine |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Emtricitabine is used in the treatment of HIV-1 infection in combination with other antiretroviral agents. SARS-CoV-2 and COVID-19: A number of Phase 2/3 clinical trials are planned to evaluate the use of emtricitabine/tenofovir to prevent SARS-COV-2 transmission to healthcare professionals. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Emtricitabine is an analogue of cytidine and is a reverse transciptase inhibitor, preventing viral replication. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04334928 | Randomized Clinical Trial for the Prevention of SARS-CoV-2 Infection (COVID-19) in Healthcare Personnel | Phase 3 Interventional | Plan Nacional sobre el Sida (PNS) | ||
| NCT04519125 | Daily Regimen of Tenofovir/Emtricitabine as Prevention for COVID-19 in Health Care Personnel in Colombia | Phase 2/Phase 3 Interventional | Hospital Universitario San Ignacio | ||
| NCT04405271 | TAF/FTC for Pre-exposure Prophylaxis of COVID-19 in Healthcare Workers (CoviPrep Study) | Phase 3 Interventional | Hospital Italiano de Buenos Aires | ||
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |