GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
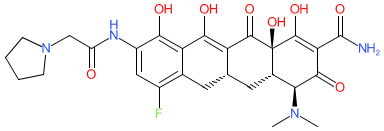
Synonyms: compound 17j [PMID: 2148514] | TP-434 | TP434 | Xerava®
eravacycline is an approved drug (EMA & FDA (2018))
Compound class:
Synthetic organic
Comment: Eravacycline is a parenteral tetracycline antibacterial.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Eravacycline received EMA and FDA approval in 2018 as a treatment for complicated intra-abdominal infections (cIAIs). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01978938 | Efficacy and Safety Study of Eravacycline Compared With Levofloxacin in Complicated Urinary Tract Infections | Phase 3 Interventional | Tetraphase Pharmaceuticals, Inc. | ||
| NCT03032510 | Efficacy and Safety Study of Eravacycline Compared With Ertapenem in Participants With Complicated Urinary Tract Infections | Phase 3 Interventional | La Jolla Pharmaceutical Company | ||
| NCT01844856 | Efficacy and Safety Study of Eravacycline Compared With Ertapenem in Complicated Intra-abdominal Infections | Phase 3 Interventional | Tetraphase Pharmaceuticals, Inc. | Eravacycline demonstrated noninferiority to ertapenem as a treatment for cIAIs in this study. | 1,3 |
| NCT02784704 | Efficacy and Safety Study of Eravacycline Compared With Meropenem in Complicated Intra-abdominal Infections | Phase 3 Interventional | La Jolla Pharmaceutical Company | Eravacycline demonstrated noninferiority to meropenem as a treatment for cIAIs (including infections caused by resistant pathogens) in this study. | 2-3 |
External links  |
|
For extended ADME data see the following: Drugs.com |