GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
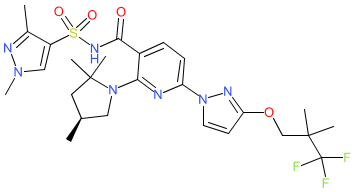
Synonyms: Compound 1 [WO2018107100A1] | VX-445
elexacaftor is an approved drug (FDA (2019))
Compound class:
Synthetic organic
Comment: Elexacaftor (VX-445) is a next-generation CFTR corrector type agent. Like its predecessor tezacaftor, elexacaftor is designed to improve CFTR protein processing and trafficking in the presence of the F508del mutation. Elexacaftor and tezacaftor CFTR corrector activities have synergistic effects [6,9]. As such a triple combination therapeutic with tezacaftor, izacaftor (a CFTR potentiator that increases CFTR channel gating activity) and elexacaftor increases CFTR function beyond that achieved by current standard of care with tezacaftor/izacaftor, and improves clinical symptoms in patients with cystic fibrosis with one or two F508del alleles [5].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Abela AR, Alcacio T, Anderson C, Angell PT, Baek M, Clemens JJ, Cleveland T, Ferris LA, Grootenhuis PDJ, Gross RS et al.. (2018)
Modulator of cystic fibrosis transmembrane conductance regulator, pharmaceutical compositions, methods of treatment, and process for making the modulator. Patent number: WO2018107100A1. Assignee: Vertex Pharmaceuticals. Priority date: 09/12/2016. Publication date: 14/04/2019. |
|
2. Drévillon L, Tanguy G, Hinzpeter A, Arous N, de Becdelièvre A, Aissat A, Tarze A, Goossens M, Fanen P. (2011)
COMMD1-mediated ubiquitination regulates CFTR trafficking. PLoS One, 6 (3): e18334. [PMID:21483833] |
|
3. Fernández Massó JR, Oliva Argüelles B, Tejeda Y, Astrada S, Garay H, Reyes O, Delgado-Roche L, Bollati-Fogolín M, Vallespí MG. (2013)
The Antitumor Peptide CIGB-552 Increases COMMD1 and Inhibits Growth of Human Lung Cancer Cells. J Amino Acids, 2013: 251398. [PMID:23401744] |
|
4. Gomez Rodriguez Y, Oliva Arguelles B, Riera-Romo M, Fernandez-De-Cossio J, Garay HE, Fernandez Masso J, Guerra Vallespi M. (2022)
Synergic effect of anticancer peptide CIGB-552 and Cisplatin in lung cancer models. Mol Biol Rep, 49 (4): 3197-3212. [PMID:35094208] |
|
5. Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, Mall MA, Welter JJ, Ramsey BW, McKee CM et al.. (2019)
Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet, 394 (10212): 1940-1948. [PMID:31679946] |
|
6. Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, Ramsey BW, Rowe SM, Sass LA, Tullis E et al.. (2018)
VX-445-Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N Engl J Med, 379 (17): 1612-1620. [PMID:30334692] |
|
7. Kolski-Andreaco A, Taiclet S, Myerburg MM, Sembrat J, Bridges RJ, Straub AC, Wills ZP, Butterworth MB, Devor DC. (2024)
Potentiation of BKCa channels by cystic fibrosis transmembrane conductance regulator correctors VX-445 and VX-121. J Clin Invest, 134 (16). [PMID:38954478] |
|
8. Tanguy G, Drévillon L, Arous N, Hasnain A, Hinzpeter A, Fritsch J, Goossens M, Fanen P. (2008)
CSN5 binds to misfolded CFTR and promotes its degradation. Biochim Biophys Acta, 1783 (6): 1189-99. [PMID:18267124] |
|
9. Veit G, Roldan A, Hancock MA, Da Fonte DF, Xu H, Hussein M, Frenkiel S, Matouk E, Velkov T, Lukacs GL. (2020)
Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight, 5 (18). [PMID:32853178] |
|
10. Veit G, Vaccarin C, Lukacs GL. (2021)
Elexacaftor co-potentiates the activity of F508del and gating mutants of CFTR. J Cyst Fibros, 20 (5): 895-898. [PMID:33775603] |