GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Enzyme Reaction
- Substrates and Reaction Kinetics
- Cofactors
- Inhibitors
- Tissue Distribution
- Clinically-Relevant Mutations and Pathophysiology
- Biologically Significant Variants
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 2 | 417 | 8p23.1 | FDFT1 | farnesyl-diphosphate farnesyltransferase 1 | 30 |
| Mouse | 2 | 416 | 14 33.24 cM | Fdft1 | farnesyl diphosphate farnesyl transferase 1 | |
| Rat | 2 | 416 | 15p12 | Fdft1 | farnesyl diphosphate farnesyl transferase 1 | |
Previous and Unofficial Names  |
| farnesyltransferase | FDFT1 | presqualene synthase | presqualene-diphosphate synthase | SQS | squalene synthetase | SSase | FPP:FPP farnesyltransferase |
Database Links  |
|
| Alphafold | P37268 (Hs), P53798 (Mm), Q02769 (Rn) |
| BRENDA | 2.5.1.21 |
| CATH/Gene3D | 1.10.600.10 |
| ChEMBL Target | CHEMBL3338 (Hs), CHEMBL4778 (Mm), CHEMBL3815 (Rn) |
| Ensembl Gene | ENSG00000079459 (Hs), ENSMUSG00000021273 (Mm), ENSRNOG00000021314 (Rn) |
| Entrez Gene | 2222 (Hs), 14137 (Mm), 29580 (Rn) |
| Human Protein Atlas | ENSG00000079459 (Hs) |
| KEGG Enzyme | 2.5.1.21 |
| KEGG Gene | hsa:2222 (Hs), mmu:14137 (Mm), rno:29580 (Rn) |
| OMIM | 184420 (Hs) |
| Pharos | P37268 (Hs) |
| RefSeq Nucleotide | NM_004462 (Hs), NM_010191 (Mm), NM_019238 (Rn) |
| RefSeq Protein | NP_004453 (Hs), NP_034321 (Mm), NP_114028 (Rn) |
| SynPHARM |
81930 (in complex with compound 15a [PMID: 22464687]) 6998 (in complex with zaragozic acid A) 6997 (in complex with zaragozic acid A) |
| UniProtKB | P37268 (Hs), P53798 (Mm), Q02769 (Rn) |
| Wikipedia | FDFT1 (Hs) |
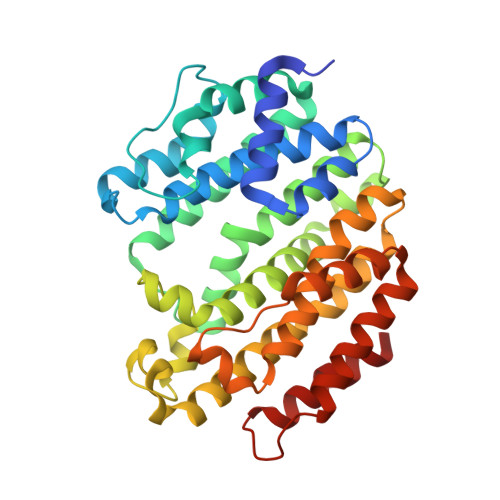
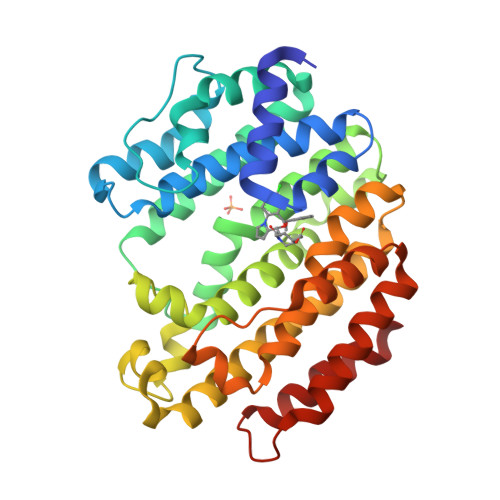
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Substrates and Reaction Kinetics  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cofactors  |
||||||||||||||||
|
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Some of the inhibitors above have been selected as representative structures from sets of stucturally similar compounds with bioactivity data at this target on ChEMBLdb.
Click here for a summary of the ChEMBL bioactivity data
![]()
Tissue Distribution 
|
||||||||
|
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||
|
||||||||
Biologically Significant Variants 
|
||||||||
|
References
1. Bergstrom JD, Kurtz MM, Rew DJ, Amend AM, Karkas JD, Bostedor RG, Bansal VS, Dufresne C, VanMiddlesworth FL, Hensens OD. (1993) Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc Natl Acad Sci USA, 90 (1): 80-4. [PMID:8419946]
2. Biller SA, Abt JW, Pudzianowski AT, Rich LC, Slusarchyk DA, Ciosek Jr CP. (1993) Aromatic isosteres as conformational probes for an isoprenyl subunit: application to inhibitors of squalene synthase. Bioorg Med Chem Lett, 3 (4): 595-600. DOI: 10.1016/S0960-894X(01)81236-1
3. Biller SA, Sofia MJ, DeLange B, Forster C, Gordon EM, Harrity T, Rich LC, Ciosek Jr CP. (1991) The First Potent Inhibitor of Squalene Synthase: A Profound Contribution of an Ether Oxygen to Inhibitor-Enzyme Interaction. J Am Chem Soc, 113 (22): 8522-8524. DOI: 10.1021/ja00022a050
4. Brinkman JA, Damon RE, Fell JB, Perez LB, Scallen TJ, Vedamanda TR. (1996) Squalene synthase inhibitors: isosteric replacements of the farnesyl chain of benzyl farnesyl amine. Bioorg Med Chem Lett, 6 (21): 2491-2494. DOI: 10.1016/0960-894X(96)00470-2
5. Brown GR, Butlin RJ, Chapman S, Eakin MA, Foubister AJ, Freeman S, Griffiths D, Harrison PJ, Johnson MC, Mallion KB. (1995) Phenoxypropylamines: a new series of squalene synthase inhibitors. J Med Chem, 38 (21): 4157-60. [PMID:7473541]
6. Brown GR, Clarke DS, Foubister AJ, Freeman S, Harrison PJ, Johnson MC, Mallion KB, McCormick J, McTaggart F, Reid AC et al.. (1996) Synthesis and activity of a novel series of 3-biarylquinuclidine squalene synthase inhibitors. J Med Chem, 39 (15): 2971-9. [PMID:8709131]
7. Brown GR, Foubister AJ, Freeman S, McTaggart F, Mirrlees DJ, Reid AC, Smith GJ, Taylor MJ, Thomason DA, Whittamore PRO. (1997) Novel optimised quinuclidine squalene synthase inhibitors. Bioorg Med Chem Lett, 7 (5): 597-600. DOI: 10.1016/S0960-894X(97)00053-X
8. Cammerer SB, Jimenez C, Jones S, Gros L, Lorente SO, Rodrigues C, Rodrigues JC, Caldera A, Ruiz Perez LM, da Souza W et al.. (2007) Quinuclidine derivatives as potential antiparasitics. Antimicrob Agents Chemother, 51 (11): 4049-61. [PMID:17709461]
9. Do R, Paré G, Montpetit A, Hudson TJ, Gaudet D, Engert JC. (2008) K45R variant of squalene synthase increases total cholesterol levels in two study samples from a French Canadian population. Hum Mutat, 29 (5): 689-94. [PMID:18350552]
10. Dufresne C, Wilson KE, Zink D, Smith J, Bergstrom JD, Kurtz M, Rew D, Nallin M, Jenkins R, Bartizal K, Trainor C, Bills G, Meinz M, Huang L, Onishi J, Milligan J, Mojena M, Pelaez F. (1992) The Isolation and Structure Elucidation of Zaragozic Acid C, a Novel Potent Squalene Synthase Inhibitor. Tetrahedron, 48 (41): 10221-10226.
11. Fung AK, Baker WR, Fakhoury S, Stein HH, Cohen J, Donner BG, Garvey DS, Spina KP, Rosenberg SH. (1997) (1 alpha, 2 beta, 3 beta, 4 alpha)-1,2-bis[N-propyl-N-(4-phenoxybenzyl) amino]carbonyl]cyclobutane-3,4-dicarboxylic acid (A-87049): a novel potent squalene synthase inhibitor. J Med Chem, 40 (14): 2123-5. [PMID:9216829]
12. Gotteland JP, Brunel I, Gendre F, Désiré J, Delhon A, Junquéro D, Oms P, Halazy S. (1995) (Aryloxy)methylsilane derivatives as new cholesterol biosynthesis inhibitors: synthesis and hypocholesterolemic activity of a new class of squalene epoxidase inhibitors. J Med Chem, 38 (17): 3207-16. [PMID:7650673]
13. Harris GH, Dufresne C, Joshua H, Koch LA, Zink DL, Salmon PM, Göklen KE, Kurtz MM, Rew DJ, Bergstrom JD, Wilson KE. (1995) Isolation, structure determination and squalene synthase activity of L-731,120 and L-731,128, alkyl citrate analogs of zaragozic acids A and B. Bioorg Med Chem Lett, 5 (20): 2403-2408.
14. Ichikawa M, Ohtsuka M, Ohki H, Haginoya N, Itoh M, Sugita K, Usui H, Suzuki M, Terayama K, Kanda A. (2012) Discovery of novel tricyclic compounds as squalene synthase inhibitors. Bioorg Med Chem, 20 (9): 3072-93. [PMID:22464687]
15. Ishihara T, Kakuta H, Moritani H, Ugawa T, Yanagisawa I. (2004) Synthesis and biological evaluation of novel propylamine derivatives as orally active squalene synthase inhibitors. Bioorg Med Chem, 12 (22): 5899-908. [PMID:15498666]
16. Iwasawa Y, Hayashi M, Nomoto T, Shibata J, Mitsuya M, Hirota K, Yonemoto M, Kamei T, Miura K, Tomimoto K. (1995) Synthesis and biological activity of J-104,118, a novel, potent inhibitor of squalene synthase. Bioorg Med Chem Lett, 5 (17): 1989-1994.
17. Iwasawa Y, Shibata J, Mitsuya M, Masaki H, Hayashi M, Kanno T, Sawasaki Y, Hisaka A, Kamei T, Tomimoto K. (1996) J-104,123, a novel and orally-active inhibitor of squalene synthase: Stereoselective synthesis and cholesterol lowering effects in dogs. Bioorg Med Chem Lett, 6 (4): 463-466.
18. Jiang G, McKenzie TL, Conrad DG, Shechter I. (1993) Transcriptional regulation by lovastatin and 25-hydroxycholesterol in HepG2 cells and molecular cloning and expression of the cDNA for the human hepatic squalene synthase. J Biol Chem, 268 (17): 12818-24. [PMID:7685352]
19. Kourounakis AP, Charitos C, Rekka EA, Kourounakis PN. (2008) Lipid-lowering (hetero)aromatic tetrahydro-1,4-oxazine derivatives with antioxidant and squalene synthase inhibitory activity. J Med Chem, 51 (18): 5861-5. [PMID:18754614]
20. Lin FY, Liu YL, Li K, Cao R, Zhu W, Axelson J, Pang R, Oldfield E. (2012) Head-to-head prenyl tranferases: anti-infective drug targets. J Med Chem, 55 (9): 4367-72. [PMID:22486710]
21. Liu CI, Jeng WY, Chang WJ, Ko TP, Wang AH. (2012) Binding modes of zaragozic acid A to human squalene synthase and staphylococcal dehydrosqualene synthase. J Biol Chem, 287 (22): 18750-7. [PMID:22474324]
22. Lolli ML, Rolando B, Tosco P, Chaurasia S, Di Stilo A, Lazzarato L, Gorassini E, Ferracini R, Oliaro-Bosso S, Fruttero R et al.. (2010) Synthesis and preliminary pharmacological characterisation of a new class of nitrogen-containing bisphosphonates (N-BPs). Bioorg Med Chem, 18 (7): 2428-38. [PMID:20299227]
23. Magnin DR, Biller SA, Chen Y, Dickson JK, Fryszman OM, Lawrence RM, Logan JV, Sieber-McMaster ES, Sulsky RB, Traeger SC et al.. (1996) alpha-Phosphonosulfonic acids: potent and selective inhibitors of squalene synthase. J Med Chem, 39 (3): 657-60. [PMID:8576905]
24. Magnin DR, Biller SA, Dickson JK, Logan JV, Lawrence RM, Chen Y, Sulsky RB, Ciosek CP, Harrity TW, Jolibois KG. (1995) 1,1-Bisphosphonate squalene synthase inhibitors: interplay between the isoprenoid subunit and the diphosphate surrogate. J Med Chem, 38 (14): 2596-605. [PMID:7629799]
25. Miki T, Kori M, Mabuchi H, Tozawa R, Nishimoto T, Sugiyama Y, Teshima K, Yukimasa H. (2002) Synthesis of novel 4,1-benzoxazepine derivatives as squalene synthase inhibitors and their inhibition of cholesterol synthesis. J Med Chem, 45 (20): 4571-80. [PMID:12238936]
26. Overhand M, Pieterman E, Cohen LH, Valentijn ARPM, van der Marel GA, van Boom JH. (1997) Synthesis of triphosphonate analogues of farnesyl pyrophosphate. Inhibitors of squalene synthase and protein:farnesyl transferase. Bioorg Med Chem Lett, 7 (18): 2435-2440.
27. Ponpipom MM, Girotra NN, Bugianesi RL, Roberts CD, Berger GD, Burk RM, Marquis RW, Parsons WH, Bartizal KF, Bergstom JD. (1994) Structure-activity relationships of C1 and C6 side chains of zaragozic acid A derivatives. J Med Chem, 37 (23): 4031-51. [PMID:7966163]
28. Prashad M. (1993) Amidinium cation as a mimic of allylic carbocation: synthesis and squalene synthetase inhibitory activity of an amidinium analog of a carbocation intermediate. J Med Chem, 36 (5): 631-2. [PMID:8496942]
29. Prashad M, Kathawala FG, Scallen T. (1993) N-(arylalkyl)farnesylamines: new potent squalene synthetase inhibitors. J Med Chem, 36 (10): 1501-4. [PMID:8496919]
30. Schechter I, Conrad DG, Hart I, Berger RC, McKenzie TL, Bleskan J, Patterson D. (1994) Localization of the squalene synthase gene (FDFT1) to human chromosome 8p22-p23.1. Genomics, 20 (1): 116-8. [PMID:8020937]
31. Sealey-Cardona M, Cammerer S, Jones S, Ruiz-Pérez LM, Brun R, Gilbert IH, Urbina JA, González-Pacanowska D. (2007) Kinetic characterization of squalene synthase from Trypanosoma cruzi: selective inhibition by quinuclidine derivatives. Antimicrob Agents Chemother, 51 (6): 2123-9. [PMID:17371809]
32. Seiki S, Frishman WH. (2009) Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: a new therapeutic approach to treatment of hypercholesterolemia. Cardiol Rev, 17 (2): 70-6. [PMID:19367148]
33. Sharratt PJ, Hutson JL, Inglis GGA, Lester MG, Procopiou PA, Watson NS. (1994) Structurally simplified squalestatins: monocyclic 1,3-dioxane analogues. Bioorg Med Chem Lett, 4 (5): 661-666.
34. Shechter I, Gu P, Jiang G, Onofrey TJ, Cann RO, Castro A, Spencer TA. (1996) Sulfobetaine zwitterionic inhibitors of squalene synthase. Bioorg Med Chem Lett, 6 (21): 2585-2588.
35. Shechter I, Klinger E, Rucker ML, Engstrom RG, Spirito JA, Islam MA, Boettcher BR, Weinstein DB. (1992) Solubilization, purification, and characterization of a truncated form of rat hepatic squalene synthetase. J Biol Chem, 267 (12): 8628-35. [PMID:1569107]
36. Shen W, Garvey DS, Cohen J, Stein H, Rosenberg SH. (1998) Cyclopentanedi- and tricarboxylic acids as squalene synthase inhibitors: syntheses and evaluation. Bioorg Med Chem Lett, 8 (8): 891-6. [PMID:9871507]
37. Soltis DA, McMahon G, Caplan SL, Dudas DA, Chamberlin HA, Vattay A, Dottavio D, Rucker ML, Engstrom RG, Cornell-Kennon SA. (1995) Expression, purification, and characterization of the human squalene synthase: use of yeast and baculoviral systems. Arch Biochem Biophys, 316 (2): 713-23. [PMID:7864626]
38. Song Y, Lin FY, Yin F, Hensler M, Rodrígues Poveda CA, Mukkamala D, Cao R, Wang H, Morita CT, González Pacanowska D et al.. (2009) Phosphonosulfonates are potent, selective inhibitors of dehydrosqualene synthase and staphyloxanthin biosynthesis in Staphylococcus aureus. J Med Chem, 52 (4): 976-88. [PMID:19191557]
39. Song Y, Liu CI, Lin FY, No JH, Hensler M, Liu YL, Jeng WY, Low J, Liu GY, Nizet V et al.. (2009) Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: in vitro, in vivo, and crystallographic results. J Med Chem, 52 (13): 3869-80. [PMID:19456099]
40. Thompson JF, Danley DE, Mazzalupo S, Milos PM, Lira ME, Harwood HJ. (1998) Truncation of human squalene synthase yields active, crystallizable protein. Arch Biochem Biophys, 350 (2): 283-90. [PMID:9473303]
41. Wattanasin S, Boettcher BR, Scallen T. (1997) N-Hydroxyglycine derivatives as novel inhibitors of squalene synthase. Bioorg Med Chem Lett, 7 (23): 3039-3044.