GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
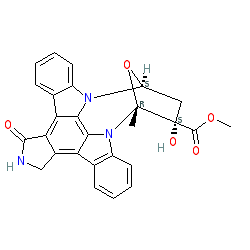
Synonyms: K252a | ST50826373 | staurosporine aglycone
Compound class:
Natural product
Comment: K252a is an alkaloid isolated from Nocardiopisis soil fungi. It is an analogue of staurosporine. There is some ambiguity surrounding the exact stereochemistry of this compound, with an alternative representation being CID 3035817.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011)
Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377] |
|
2. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013)
A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362] |
|
3. Hashimoto Y, Nakayama T, Teramoto T, Kato H, Watanabe T, Kinoshita M, Tsukamoto K, Tokunaga K, Kurokawa K, Nakanishi S. (1991)
Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative, KT5926. Biochem Biophys Res Commun, 181 (1): 423-9. [PMID:1659814] |
|
4. Hudkins RL, Johnson NW, Angeles TS, Gessner GW, Mallamo JP. (2007)
Synthesis and mixed lineage kinase activity of pyrrolocarbazole and isoindolone analogs of (+)K-252a. J Med Chem, 50 (3): 433-41. [PMID:17266195] |
|
5. Klaeger S, Heinzlmeir S, Wilhelm M, Polzer H, Vick B, Koenig PA, Reinecke M, Ruprecht B, Petzoldt S, Meng C et al.. (2017)
The target landscape of clinical kinase drugs. Science, 358 (6367). [PMID:29191878] |
|
6. Lazareno S, Popham A, Birdsall NJ. (2000)
Allosteric interactions of staurosporine and other indolocarbazoles with N-[methyl-(3)H]scopolamine and acetylcholine at muscarinic receptor subtypes: identification of a second allosteric site. Mol Pharmacol, 58 (1): 194-207. [PMID:10860942] |