GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
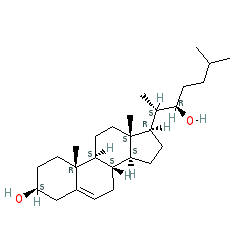
Synonyms: 22β-hydroxycholesterol | 22R-OHC
Compound class:
Metabolite
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Deng R, Yang D, Yang J, Yan B. (2006)
Oxysterol 22(R)-hydroxycholesterol induces the expression of the bile salt export pump through nuclear receptor farsenoid X receptor but not liver X receptor. J Pharmacol Exp Ther, 317 (1): 317-25. [PMID:16371446] |
|
2. Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. (1996)
An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature, 383 (6602): 728-31. [PMID:8878485] |
|
3. Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. (1997)
Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem, 272 (6): 3137-40. [PMID:9013544] |