GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
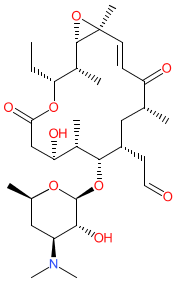
Synonyms: Antibiotic 67-694 | Antibiotic 67694 | juvenimicin A3 | rosamicin | SCH-14947 | SCH14947
Compound class:
Natural product
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Hatano K, Higashide E, Shibata M. (1976)
Studies on juvenimicin, a new antibiotic. I. Taxonomy, fermentation and antimicrobial properties. J Antibiot (Tokyo), 29 (11): 1163-70. [PMID:993103] |
|
2. Kishi T, Harada S, Yamana H, Miyake A. (1976)
Studies on juvenimicin, a new antibiotic. II. Isolation, chemical characterization and structures. J Antibiot (Tokyo), 29 (11): 1171-81. [PMID:993104] |
|
3. McFarland JW, Hecker SJ, Jaynes BH, Jefson MR, Lundy KM, Vu CB, Glazer EA, Froshauer SA, Hayashi SF, Kamicker BJ et al.. (1997)
Repromicin derivatives with potent antibacterial activity against Pasteurella multocida. J Med Chem, 40 (6): 1041-5. [PMID:9083494] |
|
4. Wagman GH, Waitz JA, Marquez J, Murawaski A, Oden EM, Testa RT, Weinstein MJ. (1972)
A new Micromonospora-produced macrolide antibiotic, rosamicin. J Antibiot (Tokyo), 25 (11): 641-6. [PMID:4647834] |
|
5. Waitz JA, Drube CG, Moss Jr EL, Weinstein MJ. (1972)
Biological studies with rosamicin, a new Micromonospora-produced macrolide antibiotic. J Antibiot (Tokyo), 25 (11): 647-52. [PMID:4486485] |