|
Synonyms: Ycanth® (0.7% solution, single-use topical applicator)
cantharidin is an approved drug (FDA (2023))
Compound class:
Natural product or derivative
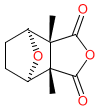
Comment: Cantharidin is a terpenoid substance that is secreted by insects such as male blister beetles. It is also synthetically manufactured. Cantharidin causes skin blisters in humans, and can be highly toxic if ingested in sufficient quantity.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03017846 | Safety and Efficacy of Topical Cantharidin for the Treatment of Molluscum Contagiosum, Phase 2 | Phase 2 Interventional | Montefiore Medical Center | ||
| NCT03377803 | Cantharidin Application in Molluscum Patients | Phase 3 Interventional | Verrica Pharmaceuticals Inc. | ||
| NCT03981822 | A Placebo-Controlled Study Using VP-102 in the Treatment of External Genital Warts | Phase 2 Interventional | Verrica Pharmaceuticals Inc. | ||