GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
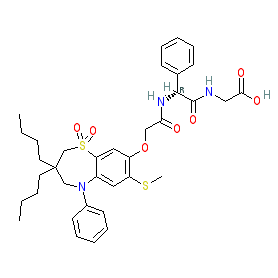
Synonyms: 2-[[(2R)-2-[[2-[(3,3-dibutyl-7-methylsulfanyl-1,1-dioxo-5-phenyl-2,4-dihydro-1λ6,5-benzothiazepin-8-yl)oxy]acetyl]amino]-2-phenylacetyl]amino]acetic acid | A3309 | AJG533 | AZD-7806 | AZD7806 | Goofice®
elobixibat is an approved drug (Japan (2018))
Compound class:
Synthetic organic
Comment: Elobixibat is an orally available inhibitor of the ileal bile acid transporter (IBAT or ASBT), which is the protein product of the SLC10A2 gene. It is being evaluated for clinical utility in the management of chronic constipation [1,3,6]. The chemical structure is claimed as Example 14 in Albireo Ab's patent US20130225511A1 [2] (we have chosen this iteration of the patent as it has both chemical structure images and data included in online versions).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Elobixibat is approved in Japan (trade name Goofice®) for the treatment of chronic constipation. Phase 3 clinical trial results from Japanese studies are published in [4] and [5]. Elobixibat was found to be effective in resolving constipation and was well tolerated in the short- and long-term. Elobixibat was also investiagted in nonalcoholic steatohepatitis (NASH) and nonalcoholic fatty liver disease (NAFLD), with the hypothesis being that ASBT inhibition might improve the liver damage (scarring and inflammation) and disordered levels of bile acids, fat and glucose, in these conditions. |
Mechanism Of Action and Pharmacodynamic Effects  |
| Elobixibat increases intracolonic concentrations of endogenous bile acids and subsequently enhances colonic secretion and improves gut motility [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04235205 | Efficacy and Safety of Elobixibat in Combination With Cholestyramine for Nonalcoholic Fatty Liver Disease | Phase 2 Interventional | Yokohama City University | ||
| NCT01038687 | Effect of Ileal Bile Acid Transporter Inhibitor in Functional Constipation | Phase 2 Interventional | Mayo Clinic | ||
| NCT04006145 | A Phase 2 Study of Elobixibat in Adults With NAFLD or NASH | Phase 2 Interventional | Albireo | Albireo terminated development of elobixibat for NASH and NAFLD, as no improvement in liver enzyme levels or reduction in liver fat that would suggest clinical benefit in NASH, was detected by this trial. | |