GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
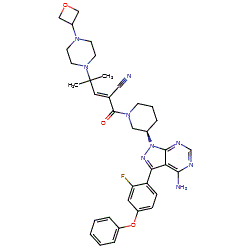
Synonyms: Example 31 [WO2014039899] | PRN-1008 | PRN1008 | Wayrilz®
rilzabrutinib is an approved drug
Compound class:
Synthetic organic
Comment: Rilzabrutinib (PRN1008) is an oral, reversible covalent inhibitor of Bruton's tyrosine kinase (BTK) [1]. One possible structure is claimed as Example 31 in Principia Biopharma patent WO2014039899 [2] and this is depicted in PubChem CID 118325989 as the R form without E/Z specification. We show the (E,R) structure here.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Rilzabrutinib (PRN1008) was advanced to clinical trials. Phase 1 results from healthy volunteers showed that orally administered PRN1008 was safe and well-tolerated , and that high and sustained levels of BTK occupancy were achieved in peripheral blood mononuclear cells [3]. More advanced studies include phase 3 evaluation in subjects with pemphigus vulgaris (NCT03762265), and a Phase 1/2 study in patients with immune thrombocytopenic purpura (ITP) (NCT03395210). Principia Biopharma modified the NCT03395210 protocol to add a long-term extension cohort for responders, and to facilitate the potential enrollment of additional patients to aid design of the Phase 3 study. First FDA approval was granted in August 2025, to treat persistent/chronic, treatment-resistant ITP. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03395210 | A Study of PRN1008 in Adult Patients With Immune Thrombocytopenic Purpura (ITP) | Phase 1/Phase 2 Interventional | Principia Biopharma Inc. | ||
| NCT03762265 | A Study of PRN1008 in Patients With Pemphigus | Phase 3 Interventional | Principia Biopharma Inc. | ||