GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Cibinqo® | compound 25 [PMID: 29298069] | PF-04965842 | PF04965842
abrocitinib is an approved drug (UK, Japan & EMA (2021), FDA (2022))
Compound class:
Synthetic organic
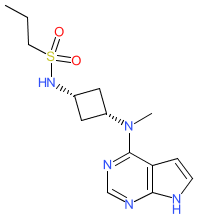
Comment: Abrocitinib (PF-04965842) is a JAK inhibitor [5]. X-ray crystal structures of PF-04965842 bound to both JAK1 and JAK2 have been deposited with the Protein DataBank (accession IDs 6BBU and 6BBV respectively). We used the SMILES string supplied with the article in which the structure was declared [5] to generate our representation of the chemical structure. The IUPAC name in abrocitinib's INN record resolves to the same StdInChIKey as our structure. PubChem CID 78323835 represents the structure without specified stereochemistry.
The JAKs are immunokinases that are molecular targets of therapeutics which are used in the treatment of immune-mediated diseases. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Nordmann TM, Anderton H, Hasegawa A, Schweizer L, Zhang P, Stadler PC, Sinha A, Metousis A, Rosenberger FA, Zwiebel M et al.. (2024)
Spatial proteomics identifies JAKi as treatment for a lethal skin disease. Nature, 635 (8040): 1001-1009. [PMID:39415009] |
|
2. Schmieder GJ, Draelos ZD, Pariser DM, Banfield C, Cox L, Hodge M, Kieras E, Parsons-Rich D, Menon S, Salganik M et al.. (2018)
Efficacy and safety of the Janus kinase 1 inhibitor PF-04965842 in patients with moderate-to-severe psoriasis: phase II, randomized, double-blind, placebo-controlled study. Br J Dermatol, 179 (1): 54-62. [PMID:28949012] |
|
3. Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, Biswas P, Valdez H, DiBonaventura M, Nduaka C et al.. (2020)
Efficacy and Safety of Abrocitinib in Patients With Moderate-to-Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol, 156 (8): 863-873. [PMID:32492087] |
|
4. Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, Bieber T, Thyssen JP, Yosipovitch G, Flohr C et al.. (2020)
Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet, 396 (10246): 255-266. [PMID:32711801] |
|
5. Vazquez ML, Kaila N, Strohbach JW, Trzupek JD, Brown MF, Flanagan ME, Mitton-Fry MJ, Johnson TA, TenBrink RE, Arnold EP et al.. (2018)
Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): A Selective JAK1 Clinical Candidate for the Treatment of Autoimmune Diseases. J Med Chem, 61 (3): 1130-1152. [PMID:29298069] |