GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
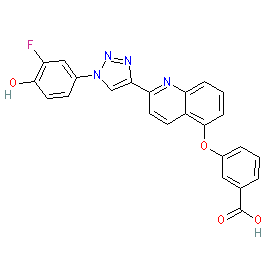
Comment: Compound 3bb is the most potent inhibitor of macrophage migration inhibitory factor (MIF) tautomerase activity identified from a SAR series by Dziedzic et al. (2015) [1]. It is referred to as quinoline 20 in [2].
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Dziedzic P, Cisneros JA, Robertson MJ, Hare AA, Danford NE, Baxter RH, Jorgensen WL. (2015)
Design, synthesis, and protein crystallography of biaryltriazoles as potent tautomerase inhibitors of macrophage migration inhibitory factor. J Am Chem Soc, 137 (8): 2996-3003. [PMID:25697265] |
|
2. Trivedi-Parmar V, Jorgensen WL. (2018)
Advances and Insights for Small Molecule Inhibition of Macrophage Migration Inhibitory Factor. J Med Chem, 61 (18): 8104-8119. [PMID:29812929] |