GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
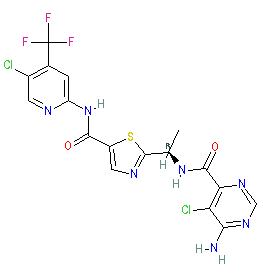
Synonyms: BIIB024 | DAY-101 | DAY101 | example 10Da [US20090036419] | MLN-2480 | MLN2480 | Ojemda® | TAK-580 | TAK580
tovorafenib is an approved drug (FDA (2024))
Compound class:
Synthetic organic
Comment: TAK-580 (MLN2480) is an investigational pan-RAF kinase inhibitor that was developed for anti-tumour potential against solid tumours, including gliomas, and common adult RAF mutant tumours (harbouring BRAF and CRAF fusions and mutations) that metastasise to the brain. It has been optimised for CNS penetration [4]. The chemical structure is claimed as example 10Da in patent US20090036419A1 [1]. Day One Biopharmaceuticals licensed this inhibitor from Sunesis Pharma in 2019, at which point it was re-named DAY101. The chemical structure of TAK-580 is identical to that which was submitted to the WHO for the INN tovorafenib (proposed INN list 126, Jan 2022).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Chen W, Cossrow J, Franklin L, Guan B, Jones JH, Kumaravel G, Lane B, Littke A, Lugovskoy A, Peng H et al.. (2012)
Compounds useful as raf kinase inhibitors. Patent number: US20090036419A1. Assignee: Sunesis Pharmaceuticals Inc. Priority date: 29/06/2007. Publication date: 23/10/2012. |
|
2. Dhillon S. (2024)
Tovorafenib: First Approval. Drugs, 84 (8): 985-993. [PMID:38967715] |
|
3. Kilburn LB, Khuong-Quang DA, Hansford JR, Landi D, van der Lugt J, Leary SES, Driever PH, Bailey S, Perreault S, McCowage G et al.. (2024)
The type II RAF inhibitor tovorafenib in relapsed/refractory pediatric low-grade glioma: the phase 2 FIREFLY-1 trial. Nat Med, 30 (1): 207-217. [PMID:37978284] |
|
4. Sun Y, Alberta JA, Pilarz C, Calligaris D, Chadwick EJ, Ramkissoon SH, Ramkissoon LA, Garcia VM, Mazzola E, Goumnerova L et al.. (2017)
A brain-penetrant RAF dimer antagonist for the noncanonical BRAF oncoprotein of pediatric low-grade astrocytomas. Neuro-oncology, 19 (6): 774-785. [PMID:28082416] |