GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: compound 24 [PMID: 21316218] | RO4987655
Compound class:
Synthetic organic
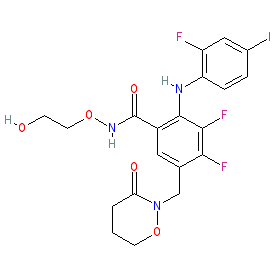
Comment: CH4987655 is an orally active allosteric MEK inhibitor that was designed as a novel cancer chemotherapeutic [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A single Phase 1 dose-escalating study to evaluate CH4987655 (RO4987655 in the trial) efficacy in patients with advanced solid tumours has been completed (NCT00817518) [3-5], and results showed promising preliminary antitumor activity. An expansion of the study in patients with advanced cancer with RAS-RAF mutations failed to show efficacy superior to other MEK inhibitors [5]. A lack of further activity suggests that development of this candidate has been discontinued. |
Mechanism Of Action and Pharmacodynamic Effects  |
| The mitogen-activated protein kinase (MAPK) pathway (the Ras/Raf/MEK/ERK signaling cascade) is one of the most important pathways involved in cell proliferation and differentiation, and abberant pathway activation driven by K-Ras or B-Raf mutations in tumours is common. Hence, drug companies have long been developing inhibitors of components of the MAPK pathway as cancer chemotherapeutics [1]. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT00817518 | A Dose-Escalating Study of RO4987655 in Patients With Advanced Solid Tumors | Phase 1 Interventional | Hoffmann-La Roche | ||