GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Compound class:
Synthetic organic
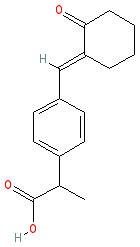
Comment: Pelubiprofen is a nonsteroidal anti-inflammatory type compound [6]. Structurally it is an aryl propionic acid derivative, like ibuprofen. The INN-defined agent is a racemic mixture of enantiomers. We show the structure without specified stereochemistry to represent the mixture. Pelubiprofen is described as a prodrug of 2-arylpropionic acid with modest selectivity for COX2 [3].
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Pelubiprofen has completed Phase 3 clinical evaluations for the management of the inflammation and pain associated with rheumatoid arthritis (NCT01781702) and chronic back pain (NCT02375633). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01781702 | Phase 3 Study of Pelubiprofen Tab. & Celebrex Cap. in Rheumatoid Arthritis Patients | Phase 3 Interventional | Daewon Pharmaceutical Co., Ltd. | ||
| NCT02375633 | Phase 3 Study of DW-330SR2 and Pelubiprofen in Chronic Back Pain Patients | Phase 3 Interventional | Daewon Pharmaceutical Co., Ltd. | ||