GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
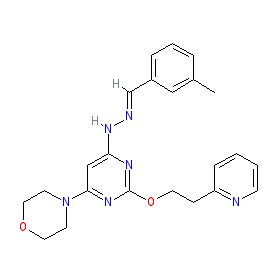
Synonyms: LAM-002A (apilimod dimesylate) | LAM002A | STA 5326 | STA-5326 | STA5326
Compound class:
Synthetic organic
Comment: Apilimod was originally identified as an inhibitor of IL-12/IL-23 production [12], but the molecular mechanism behind this biological effect was undetermined. Inhibition of IL-12/IL-23 production has application for the treatment Th1-and Th17-mediated immunologic pathologies (which are driven by IL-12 and IL-23 respectively) [11-12]. Apilimod has subsequently been identified as a selective inhibitor of the type III phosphoinostol kinase, PIKfyve [4-5].
We provide structural details for the parent molecule, but some bioactivity data may relate to use of the mesylate salt form as stipulated by the compound's USAN (see PubChem CID 115273300). PIKfyve has been reported as being required for Ebola virus (EBOV) infection of host cells [9], and indeed apilimod has anti-EBOV activity in vitro. More broad-spectrum antiviral activity against Lassa virus (LASV), Marburg virus (MARV) [8] and SARS-CoV-2 [6,10] is supported in the literature. Activity of PIKfyve is markedly modulated by SARS-CoV-2 infection in vitro, and indicates potential hijacking of phosphatidylinositol enzyme activities by the coronavirus [3]. Pharmacological inhibition of this kinase with apilimod has anti-SARS-CoV-2 activity in two model cell lines, A549-ACE2 cells (IC50 7 nM) and Vero E6 cells (IC50 80 nM), and in human iPSC-derived pneumocyte-like cells Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02594384 | A Phase I Dose Escalation Study of the Safety and Pharmacokinetics of LAM-002A In Patients With Non-Hodgkin's Lymphoma (LAM-002A/NHL) | Phase 1 Interventional | AI Therapeutics, Inc. | ||
| NCT04446377 | A Study of LAM-002A for the Prevention of Progression of COVID-19 | Phase 2 Interventional | AI Therapeutics, Inc. | ||