GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: RG7129
Compound class:
Synthetic organic
Comment: Roche progressed RG7129/RO5508887 into clinical trials in 2011 as a potent aminooxazoline inhibitor against BACE1 but not selective over BACE2. In October 2013, development ceased without explanation. The most recent peer reviewed paper, is a mouse study in combination with anti-Aβ antibody gantenerumab [3]. A patent has published with an SAR set of 84 IC50 values [1], and there are also a total of 14 PDB structures from this series published, including RO5508887 [2].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
Classification  |
|
| Compound class | Synthetic organic |
IUPAC Name  |
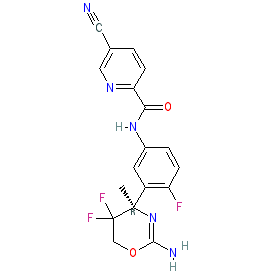
| N-[3-[(4R)-2-amino-5,5-difluoro-4-methyl-6H-1,3-oxazin-4-yl]-4-fluorophenyl]-5-cyanopyridine-2-carboxamide |
Synonyms  |
| RG7129 |
Database Links  |
|
| ChEMBL Ligand | CHEMBL2347215 |
| GtoPdb PubChem SID | 354702292 |
| PubChem CID | 53241828 |
| RCSB PDB Ligand | 6Z0 |
| Search Google for chemical match using the InChIKey | DVMUZHLUMHPCGZ-QGZVFWFLSA-N |
| Search Google for chemicals with the same backbone | DVMUZHLUMHPCGZ |
| SynPHARM | 84891 (in complex with beta-secretase 1) |
| UniChem Compound Search for chemical match using the InChIKey | DVMUZHLUMHPCGZ-QGZVFWFLSA-N |
| UniChem Connectivity Search for chemical match using the InChIKey | DVMUZHLUMHPCGZ-QGZVFWFLSA-N |
Product suppliers
View disclaimer
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖
Sponsored products disclaimer
Product supplier links are provided as a service to assist in identifying commercial suppliers of reagents that are mentioned on the IUPHAR/BPS Guide to PHARMACOLOGY database website, and do not imply their endorsement by NC-IUPHAR.
Links are provided in return for sponsorship, used to fund improvements to this database. The sponsorship account is managed and audited by the University of Edinburgh, a charitable body registered in Scotland, SC005336. If you are interested in sponsoring the database, please contact us.
✖|
BACE1-IN-1 (links to external site)
Cat. No. HY-100182 |