GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
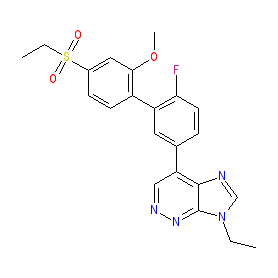
Synonyms: example 4 [WO2014091368] | PF-06372865 | PF06372865
Compound class:
Synthetic organic
Comment: Darigabat (PF-06372865) is an orally active, GABAA receptor positive allosteric modulator, that selectively targets GABAA receptors containing α2/3/5 subunits over receptors containing α1 subunits [1,3]. The α1 subunit is associated with the (undesirable) sedative effects of benzodiazepine drugs, whilst the α2/3 subunits contribute to their anxiolytic and analgesic activities and the α5 subunit to effects on memory function. The chemical structure of this compound is claimed as 'Example 4' in patent WO2014091368 [2]. Pfizer are developing PF-06372865 for clinical potential in the treatment of chronic pain [4] and photosensitive epilepsy, but have terminated development in generalized anxiety disorder.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| PF-06372865 has completed a Phase 2 clinical trial in patients with photosensitive epilepsy (NCT02564029). In this trial PF-06372865 was compared with lorazepam (as a positive control) and placebo. A Phase 2 study in patients with chronic low back pain has also been completed (NCT02262754). Click here to link to ClinicalTrials.gov's full list of PF-06372865 trials. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02262754 | PF-06372865 In Subjects With Chronic Low Back Pain | Phase 2 Interventional | Pfizer | ||
| NCT02564029 | PF-06372865 in Subjects With Photosensitive Epilepsy | Phase 2 Interventional | Pfizer | ||