GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
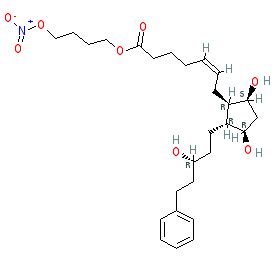
Synonyms: BOL-303259-X | NCX-116 | PF-3187207 | Vyzulta®
latanoprostene bunod is an approved drug (FDA (2017))
Compound class:
Synthetic organic
Comment: Latanoprostene bunod is a NO-donating analogue of the prostaglandin F2α agonist latanoprost (isopropyl ester) [3]. This compound benefits from the additive hypotensive actions of both the prostaglandin agonist and NO. Latanoprostene bunod is the first NO-donating prostaglandin analogue to be granted FDA marketing approval [2,4].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Several Phase 3 clinical trials evaluating latanoprostene bunod (BOL-303259-X) in subjects with ocular hypertension (esp. open-angle glaucoma) have been completed. For example, NCT01749904 [6] and NCT01749930 [5] which compared BOL-303259-X with the β-blocker timolol. Published results from both of these studies show that latanoprostene bunod had a greater intraocular pressure-lowering effect than timolol over the 3 month study period [5-6]. The drug was approved by the FDA in November 2017, for the treatment of open-angle glaucoma or ocular hypertension. |