GtoPdb is requesting financial support from commercial users. Please see our sustainability page for more information.
|
Synonyms: Anzupgo® | Corectim® | ent-60 [5] | JTE-052 | JTE052
delgocitinib is an approved drug
Compound class:
Synthetic organic
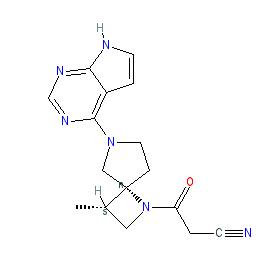
Comment: Delgocitinib (JTE-052) is an ATP-competitive pan-Janus kinase (JAK) inhibitor in development for anti-inflammatory potential [6]. The chemical structure is disclosed in [5]. The pharmacology of JTE-052 was previously reported in [6]. Based on the developer's pipeline and patent claims, and on the structure submitted to the WHO for the INN delgocitinib, JTE-052 is likely to be compound 6 as claimed in patent US20140187534 [4]. Compound 6 is the most potent (lowest IC50) optically active stereoisomer with the correct structure in the patent, although stereochemistry has not rendered unambiguously in the patent extraction, and an IUPAC name is not provided.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The developer's (Japan Tobacco Inc.) pipeline update in May 2017 listed JTE-052 as being in Phase 2 clinical development. In January 2020 topical delgocitinib was approved in Japan, for the treatment of atopic dermatitis [2], with Phase 3 results being published by Nakagawa et al. 3 months later [3]. In Europe the EMA approved delgocitinib (Anzupgo®) as a treatment for moderate/severe chronic hand eczema in September 2024, with FDA approval for this indication following in July 2025. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03683719 | Phase 2b Dose-ranging Trial to Evaluate Delgocitinib Cream 1, 3, 8, and 20 mg/g Compared to Delgocitinib Cream Vehicle Over a 16-week Treatment Period in Adult Subjects With Chronic Hand Eczema | Phase 2 Interventional | LEO Pharma | ||
| NCT02664805 | Proof of Concept, Twice Daily Applications of LEO 124249 Ointment in the Treatment of Chronic Hand Eczema | Phase 2 Interventional | LEO Pharma | 7 | |
| NCT05332366 | A Trial to Assess the Effect of Delgocitinib Cream 20 mg/g on the Molecular Signature, Safety, and Efficacy in Adults With Frontal Fibrosing Alopecia | Phase 2 Interventional | LEO Pharma | ||
| NCT05355818 | Efficacy and Safety of Delgocitinib Cream in Adolescents 12-17 Years of Age With Moderate to Severe Chronic Hand Eczema | Phase 3 Interventional | LEO Pharma | ||
| NCT06004050 | A Trial to Evaluate Efficacy, Safety, and Pharmacokinetics of Delgocitinib Cream in Chinese Adults and Adolescents with Moderate to Severe Chronic Hand Eczema | Phase 3 Interventional | LEO Pharma | ||
| NCT04871711 | Efficacy and Safety of Delgocitinib Cream in Adults With Moderate to Severe Chronic Hand Eczema | Phase 3 Interventional | LEO Pharma | 1 | |
| NCT04872101 | Efficacy and Safety of Delgocitinib Cream in Adults With Moderate to Severe Chronic Hand Eczema (DELTA 2) | Phase 3 Interventional | LEO Pharma | 1 | |